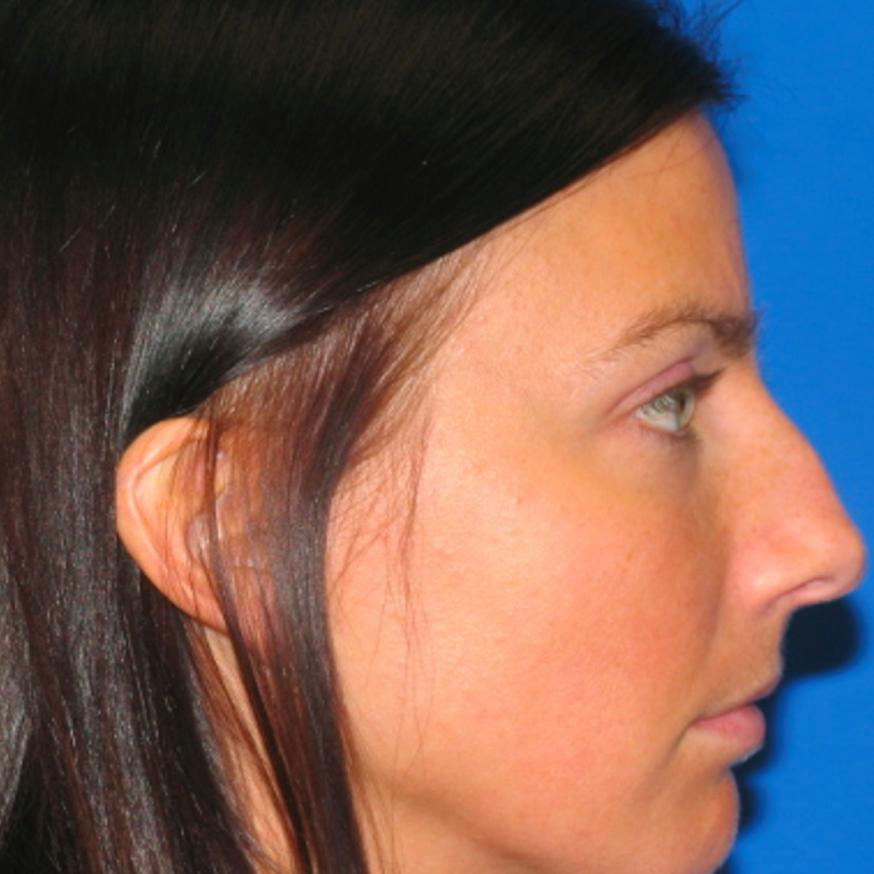
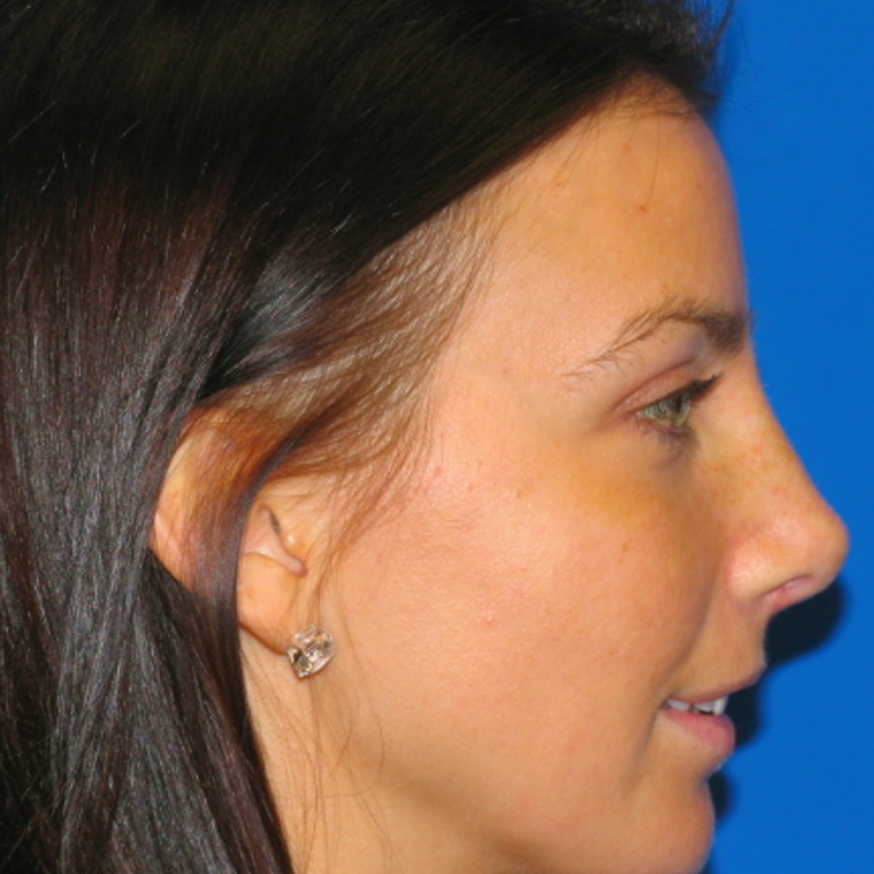
Breast Revision
Conveniently located to serve the areas of Tampa and Lakeland, FL

If you’ve had breast augmentation surgery in the past and are no longer satisfied with the results, you may be a good candidate for breast implant revision. This procedure can help you achieve the desired size, shape, and appearance of your breasts.
Contents
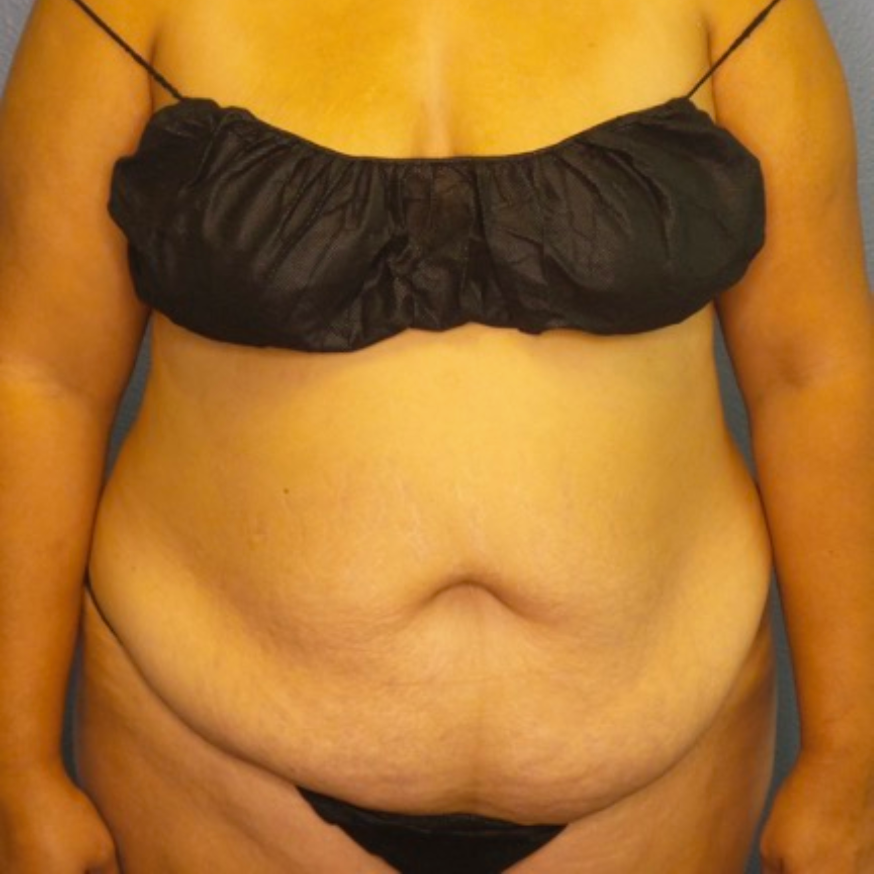
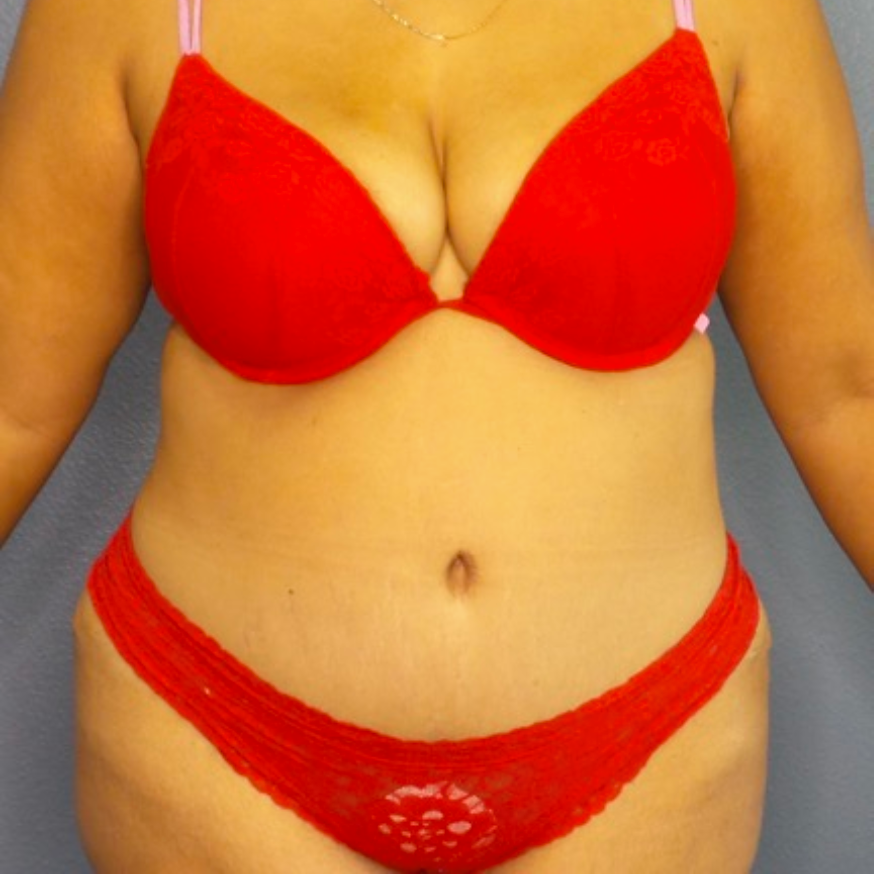
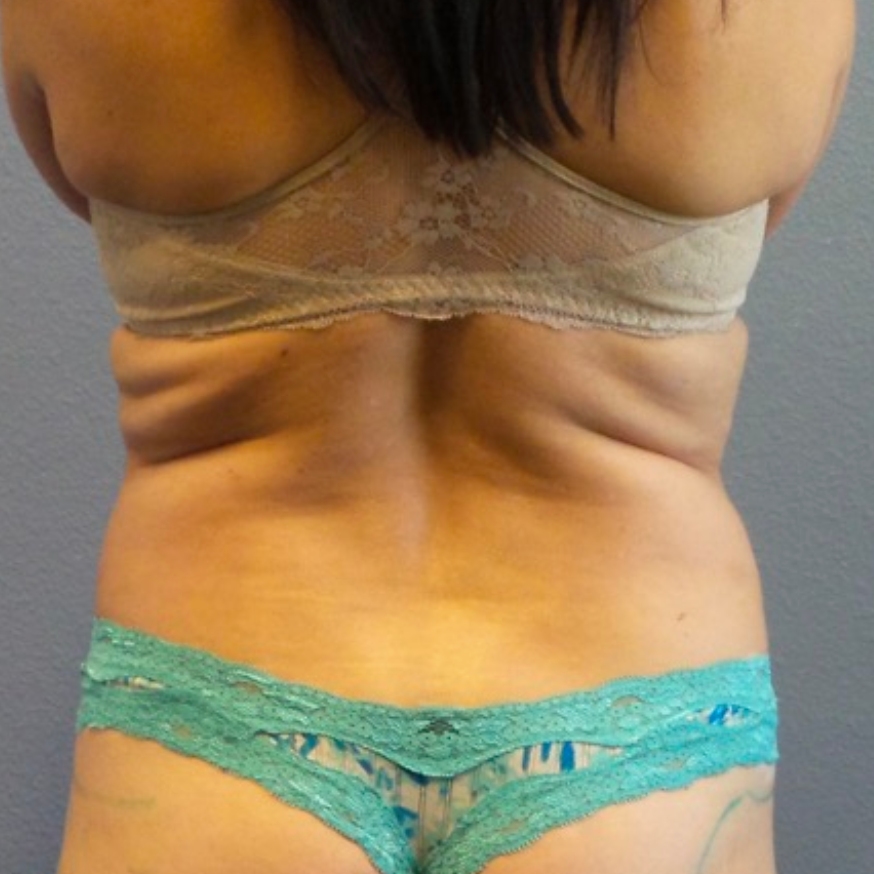
Before and After Photos
Breast implant revision involves removing or replacing the existing breast implants with new ones. It is a common procedure that is performed for a variety of reasons, including implant rupture, capsular contracture, implant malposition, and dissatisfaction with the appearance of the breasts.
Procedure
The procedure for breast implant revision varies depending on the specific needs and goals of each patient. During the consultation, your plastic surgeon will evaluate your condition and recommend the best approach for you.
In general, the procedure involves removing the existing implants and performing any necessary adjustments to the breast tissue or implant pocket. Then, new implants are inserted to achieve the desired size and shape of the breasts.
The surgeon can perform implant revision surgery under general anesthesia, and they typically complete the procedure within one to two hours. Meanwhile, recovery time varies depending on the extent of the procedure, but most patients can return to work within a week.
Breast Augmentation Repair
Breast augmentation is a popular cosmetic procedure, but there are instances where revisions and repairs are necessary. This article explores the world of breast augmentation repair, the solutions it offers, and how it can boost your confidence and enhance your appearance.
Common Reasons for Repair
Various factors may lead to the need for breast augmentation repair. Whether it’s addressing implant complications, size changes, or aesthetic preferences, understanding the specific reasons behind the repair is crucial.
Breast Implant Revision
Breast implant revision is a specialized procedure designed to correct issues that may arise after initial breast augmentation. Whether you’re experiencing implant rupture, displacement, or simply want to update your implant type or size, this procedure can help achieve your desired results.
Corrective Breast Enhancement
Corrective breast enhancement allows for tailored adjustments to your breast implants. It ensures that your implants complement your overall look, whether that involves size, shape, or other factors. It’s a personalized approach to enhance your appearance. Whether you’re seeking to restore symmetry, address aging-related changes, or simply refine your breast augmentation results, corrective breast enhancement offers a personalized and comprehensive solution to help you achieve the natural and balanced appearance you desire.
Addressing Complications
Sometimes, complications occur after breast augmentation, such as capsular contracture or asymmetry. Corrective procedures can rectify these issues, resulting in a more natural and harmonious appearance.
Benfits
Breast implant revision can offer many benefits, including:
- Improved breast symmetry and shape
- Enhanced breast size and projection
- Improved breast contour and appearance
- Correcting any complications from previous breast implant surgery
Risks
Like any surgical procedure, Implant revision surgery carries some potential risks and complications. These can include:
- Bleeding and infection
- Scarring
- Capsular contracture
- Implant rupture or deflation
- Changes in nipple sensation
Recovery and Results
Recovery after breast augmentation repair varies depending on the specific procedure performed. Generally, patients can expect some swelling and discomfort, which subside over time. The results of the repair become more evident as the healing process continues. With proper care and follow-up appointments with your surgeon, you can enjoy the long-term benefits of a successful breast augmentation repair, restoring both your confidence and physical well-being.
Choosing a Surgeon
Selecting a skilled and experienced plastic surgeon is crucial for achieving optimal results with breast implant revision surgery. Patients should research potential surgeons, view before-and-after photos, and schedule consultations to discuss their goals and concerns. A qualified surgeon will listen attentively, provide personalized recommendations, and guide patients through the entire process.
Conclusion
If you’re considering breast implant revision, it’s important to consult with a qualified plastic surgeon to discuss your goals and expectations. Our experts can help you understand the benefits and risks of the procedure and recommend the best approach for you. With proper care and attention, you can achieve the results you desire and feel confident in your appearance.
Contact
Before any repair procedure, it’s essential to consult with a board-certified plastic surgeon. They will assess your unique situation, discuss your goals, and create a personalized plan to achieve the desired results.