Breast Augmentation
Conveniently located to serve the areas of Tampa and Lakeland, FL

Breast augmentation is a cosmetic surgical procedure aimed at enhancing the size, shape, or fullness of the breasts. This procedure involves the insertion of implants under the breast tissue or chest muscle to improve the appearance of the breasts. For women, breast augmentation can be a way to achieve a more youthful, voluptuous, and confident appearance.
Contact UsContents
- 1 Before and After Photos
- 2 Procedure
- 3 Benefits
- 4 Expectations
- 5 Risks
- 6 Recommended Age
- 7 Choosing a Surgeon
- 8 Consultation
- 9 Recovery and Results
- 10 Cost
- 11 Conclusion
- 12 FAQ
- 12.1 What are the different types of tuberous breasts?
- 12.2 What medical conditions have not had safety and effectiveness established in accordance with FDA standards with respect to saline breast implantation?
- 12.3 What is wound dehiscence after breast augmentation surgery, what are the consequences, and what is the treatment?
- 12.4 Smooth Vs. Textured
- 12.5 What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Siltex Contour Profile® Spectrum® Breast Implants?
- 12.6 What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Smooth Round Spectrum® Breast Implants? What are their advantages and disadvantages?
- 12.7 What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Siltex Round Spectrum® Breast Implants? What are their advantages and disadvantages?
- 12.8 What is subglandular breast implant placement in breast augmentation?
- 12.9 Subglandular Vs. Submuscular Vs. “Dual-Plane” Breast Implant Placement
- 12.10 What is submuscular breast implant placement in breast augmentation?
- 12.11 What is breast enlargement or breast augmentation surgery?
- 12.12 Should I have breast augmentation using textured or smooth breast implants?
- 12.13 What are Titanium Coated Breast Implants?
- 12.14 Am I too old for breast enlargement or breast augmentation surgery?
- 12.15 Am I too young for breast enlargement or breast augmentation surgery?
- 12.16 What is a transumbilical breast augmentation incision?
- 12.17 What are Trilucent Breast Implants (Peanut Oil Breast Implants, Soy Oil Breast Implants, Soya Oil Breast Implants, Soybean Oil Breast Implants)?
- 12.18 Incision Type
- 12.19 What is implant exposure or breast implant extrusion after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatment?
- 12.20 Is implant removal common after breast augmentation surgery with silicone breast implants?
- 12.21 Is there a way to tell my own breast tissue from silicone breast implants during self examination?
- 12.22 Is infection common after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatment?
- 12.23 Should I have breast augmentation using silicone or saline prosthesis?
- 12.24 Is pain normal after breast augmentation surgery with silicone implants?
- 12.25 Is seroma (fluid accumulation as your body attempts to fill a potential space) common after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatme
- 12.26 Is breast tissue or skin flap death (necrosis) common after breast augmentation surgery with silicone breast implants?
- 12.27 Am I a good candidate for the use of silicone gel-filled breast implants in my breast augmentation surgery?
- 12.28 Is silicone gel-filled breast implant rupture obvious, or easily detected?
- 12.29 Are unsatisfactory results common after breast augmentation surgery with silicone breast implants?
- 12.30 Is breast tissue or skin flap death (necrosis) common after breast augmentation surgery?
- 12.31 What Were The Main Reasons For Implant Removal in The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants?
- 12.32 What Were The Complication Rates After 4 Years of Follow-up?
- 12.33 What Were The Benefits to Silicone Breast Augmentation according to the Allergan Core Study on INAMED® Silicone-Filled Breast Implants?
- 12.34 What was the followup rate in the Allergan Core Study on INAMED® Silicone-Filled Breast Implants?
- 12.35 What Were The Main Reasons For Reoperation in The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants?
- 12.36 What were the Allergan Core Study Results on INAMED® Silicone-Filled Breast Implants for primary augmentation and revision-augmentation?
- 12.37 What is the difference between silicone and saline filled breast implants?
- 12.38 Will I be able to breast feed after breast enlargement surgery with silicone breast implants?
- 12.39 Is breast tissue atrophy common after breast enlargement surgery with silicone breast implants?
- 12.40 Can calcium collections in the breast after silicone breast implant surgery be mistaken for cancer?
- 12.41 Is there an association between breast augmentation surgery with silicone breast implants and cancer, or other serious conditions?
- 12.42 What is capsular contracture after breast enlargement surgery with silicone breast implants?
- 12.43 Is chest wall deformity common after breast enlargement surgery with silicone breast implants?
- 12.44 Is there an asociation between silicone breast implants and connective tissue disease?
- 12.45 Does baby get exposed to silicone that diffuses from silicone breast implants?
- 12.46 Does the use silicone gel-filled breast implants in breast enlargement surgery affect my insurance premiums?
- 12.47 What is a periareolar incision in breast enlargement surgery?
- 12.48 What are PIP Saline Breast Implants?
- 12.49 Should I opt for Subglandular, Submuscular, or the “Dual-Plane” Breast Implant Placement?
- 12.50 What are Polyurethane Coated Breast Implants?
- 12.51 How did MHRA bring the Polyurethane Coated Breast Implant issue to plastic surgery patients?
- 12.52 How did MHRA bring the Polyurethane Coated Breast Implant issue to plastic surgery professionals?
- 12.53 What can I expect in the post-operative period following breast enlargement surgery?
- 12.54 Are there any particular questions you should ask of your plastic surgeon in consultation?
- 12.55 If I need revision breast augmentation surgery, or “touch up” breast enlargement surgery do I have to pay?
- 12.56 What Were The Main Reasons For Implant Removal in The Allergan Core Study for Revision-Augmentation with INAMED® Silicone Breast Implants?
- 12.57 What Were The Main Reasons For Reoperation in The Allergan Core Study for Revision-Augmentation with INAMED® Silicone Breast Implants?
- 12.58 Saline Vs. Silicone
- 12.59 Should I perform self breast examinations after breast enlargement surgery?
- 12.60 How can breast implants change the shape of my breasts?
- 12.61 Shaped Vs. Round
- 12.62 What are MemoryGel™ Smooth Round Moderate Profile Breast Implants? What are their advantages and disadvantages?
- 12.63 What are MemoryGel™ Smooth Round Moderate Plus Profile Breast Implants? What are their advantages and disadvantages?
- 12.64 What are Contour Profile® High Breast Implants Style 2900? What are their advantages and disadvantages?
- 12.65 What are Contour Profile® Moderate Breast Implants Style 2900? What are their advantages and disadvantages?
- 12.66 What are the various silicone implants available from Mentor?
- 12.67 What are Siltex® Round Moderate Profile Breast Implant Style 2600? What are their advantages and disadvantages?
- 12.68 What are Round Saline Breast Implants? Particularly the Smooth Round High Profile Breast Implants, Style 3000 from Mentor? What are their advantages and disadvantages?
- 12.69 What are Round Saline Breast Implants? Particularly the Smooth Round Moderate Profile Breast Implants, Style 1600 from Mentor? What are their advantages and disadvantages?
- 12.70 What are Round Saline Breast Implants? Particularly the Smooth Round Moderate Plus Profile Breast Implants, Style 2000 from Mentor? What are their advantages and disadvantages?
- 12.71 What was presented to the committee on safety of devices (MHRA)?
- 12.72 Is the need for additional surgeries (reoperations) common after breast augmentation surgery with silicone breast implants?
- 12.73 Is breast augmentation surgery the only surgery I will ever need to enhance my breasts?
- 12.74 Is the need for additional breast procedures common after breast enlargement surgery?
- 12.75 Is breast augmentation surgery painful?
- 12.76 What type of incision should I chose when undergoing breast enlargement surgery? What are the advantages and disadvantages of each?
- 12.77 Is infection common after breast enlargement surgery, what are the consequences, and what is the treatment?
- 12.78 What is an inframammary incision with respect to breast augmentation?
- 12.79 Are silicone breast implants safe?
- 12.80 Can any patient receive the latest generation cohesive or gummy-bear silicone implants?
- 12.81 Is loss of sensation from the skin of the lower pole of the breast common after breast enlargement surgery, what are the consequences, and what is the treatment?
- 12.82 Is loss of nipple sensation possible after breast augmentation surgery?
- 12.83 Is it better to use a low profile breast implant or a high profile breast prosthesis in breast enlargement surgery?
- 12.84 Does breast enlargement surgery, or do breast implants interfere with mammography?
- 12.85 Should I have a mammogram prior to have breast augmentation surgery?
- 12.86 Should I massage my breasts after breast enlargement surgery?
- 12.87 What is the McGhan style 410 breast implant, what are its features, and when should it be used?
- 12.88 What various sizes and shapes do the 410 “gummy bear” silicone gel implants come in?
- 12.89 What are MemoryGel™ Smooth Round High Profile Breast Implants? What are their advantages and disadvantages?
- 12.90 What is the thinking behind making the cohesive or gummy bear implant anatomically contoured?
- 12.91 Is it possible for “Gummy Bear Breast Implants” to rupture?
- 12.92 What are Cohesive Gel Breast Implants (“Gummy Bear Breast Implants”)?
- 12.93 Why is the cohesive “gummy bear” implant being investigated?
- 12.94 How can I tell if my cohesive gel (“gummy bear”) breast implants have ruptured, or are leaking?
- 12.95 Unfortunately, this is a question that can be answered only in speculation, without hard medical evidence. Presumably the more solid, formed nature of silicone “gummy bear” implant would not allow as
- 12.96 What are Hydrogel Breast Implants (Hyaluronic-Acid Filled Breast Implants)?
- 12.97 Besides silicone breast implants, or saline breast prostheses, what else is out there?
- 12.98 What is implant exposure or breast implant extrusion after breast enlargement surgery, what are the consequences, and what is the treatment?
- 12.99 How can the palpability of a breast implant be reduced in breast enlargement surgery?
- 12.100 What is palpable or visible breast implant rippling after breast augmentation surgery, what are the consequences, and what is the treatment?
- 12.101 What are some of the unestablished consequences of implant rupture?
- 12.102 Is breast implant rupture common after breast augmentation surgery, what are the consequences, and what is the treatment?
- 12.103 What type of implants should I ask my surgeon to use in enlarging my mammaries through breast augmentation surgery?
- 12.104 Do breast implants come with a warranty, if so what does the warranty cover, and what is excluded?
- 12.105 What type of surgical techniques are not appropriate for plastic surgeons to use in breast augmentation surgery for risk of implant shell rupture?
- 12.106 How should I care for my incisions after breast enlargement surgery?
- 12.107 Who would be considered a good candidate for breast enlargement or breast augmentation surgery?
- 12.108 What is capsular contracture after breast augmentation surgery, what are the consequences, and what is the treatment?
- 12.109 Is chest wall deformity common after breast enlargement surgery?
- 12.110 Is chronic pain common after breast enlargement surgery?
- 12.111 What is closed capsulotomy?
- 12.112 Are gummy bear implants the final word in breast augmentation?
- 12.113 Can any surgeon use the cohesive gummy bear implant?
- 12.114 Is there a way to detect cohesive gel (“gummy bear”) breast implant rupture or leak?
- 12.115 What happens if “Gummy Bear Breast Implants” tear, rupture, or leak?
- 12.116 Why aren’t any Inamed or Mentor contoured or anatomically shaped silicone gummy bear breast implants made smooth?
- 12.117 What are some of the complications of breast enlargement surgery?
- 12.118 Is there an association between breast augmentation surgery and connective tissue disease?
- 12.119 What other points should I consider when chosing silicone gel-filled breast implants to be used in my breast enlargement?
- 12.120 What is the customary cost of breast augmentation or breast enlargement surgery?
- 12.121 Is dissatisfaction with cosmetic results common after breast augmentation surgery?
- 12.122 What is “dual-plane” breast implant placement in breast augmentation?
- 12.123 Is there a way to tell my own breast tissue from saline breast implants during self examination?
- 12.124 What are the restrictions to activity after breast enlargement surgery?
- 12.125 What are the advantages and disadvantages to the use of cohesive “gummy bear” breast implants?
- 12.126 How and why do breasts shape change with age?
- 12.127 What are the various saline implants available from Allergan?
- 12.128 What are the various silicone implants available from Allergan?
- 12.129 I not happy with the size of my breasts, but I do not want breast enlargement surgery. What are my options?
- 12.130 Should my physician use a shaped or round implant in performing my breast augmentation?
- 12.131 What is an axillary incision in breast enlargement surgery?
- 12.132 Is bleeding common after breast augmentation surgery, what are the consequences, and what is the treatment?
- 12.133 Where do breasts get their shape?
- 12.134 Will there be breast skin and breast tissue changes after silicone gel-filled breast implant use in breast augmentation surgery?
- 12.135 Is breast tissue atrophy common after breast enlargement surgery?
- 12.136 Is it safe to breastfeed my child after silicone breast enlargement surgery?
- 12.137 Can calcium collections in the breast after saline or silicone breast implant surgery be mistaken for cancer?
- 12.138 Is there an association between breast augmentation surgery and cancer?
- 12.139 Am I a good candidate for saline-filled breast implants?
- 12.140 What type of anesthesia is typically used to carry out breast augmentation or breast enlargement surgery?
- 12.141 Will I be able to breast feed after breast enlargement surgery?
- 12.142 Is delayed wound healing common after breast enlargement surgery?
- 12.143 Is abscess formation common after breast augmentation surgery with silicone breast implants, what are the consequences, and what is the treatment?
- 12.144 When should silicone gel-filled breast implants not be used in breast enlargement surgery?
- 13 Sources
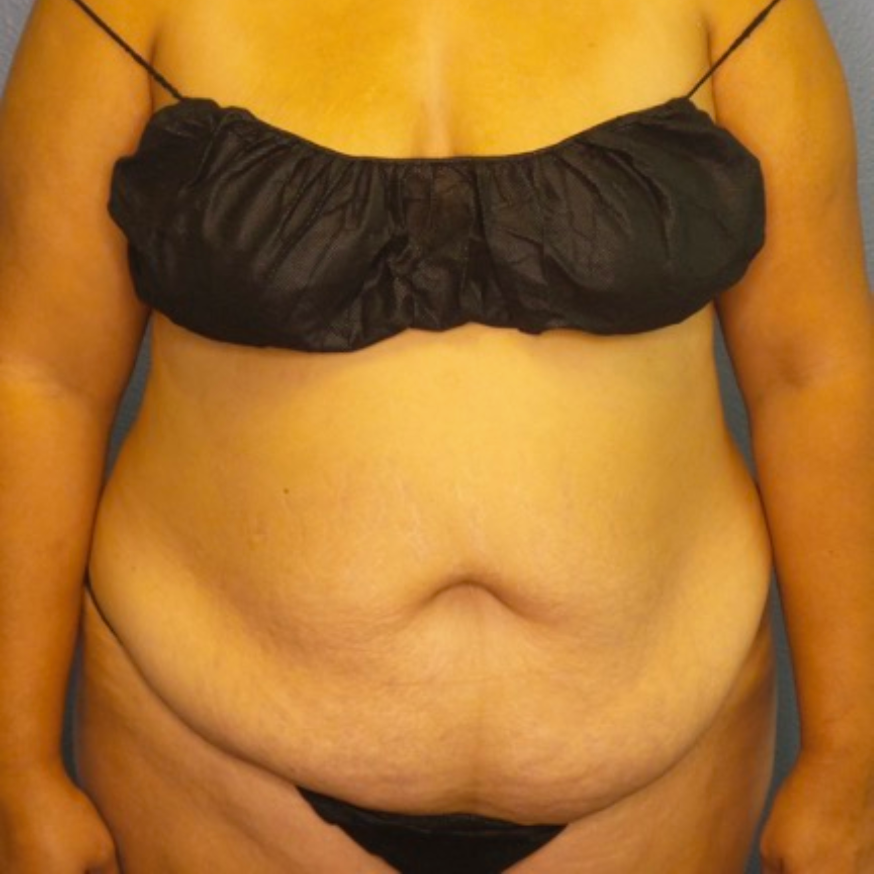
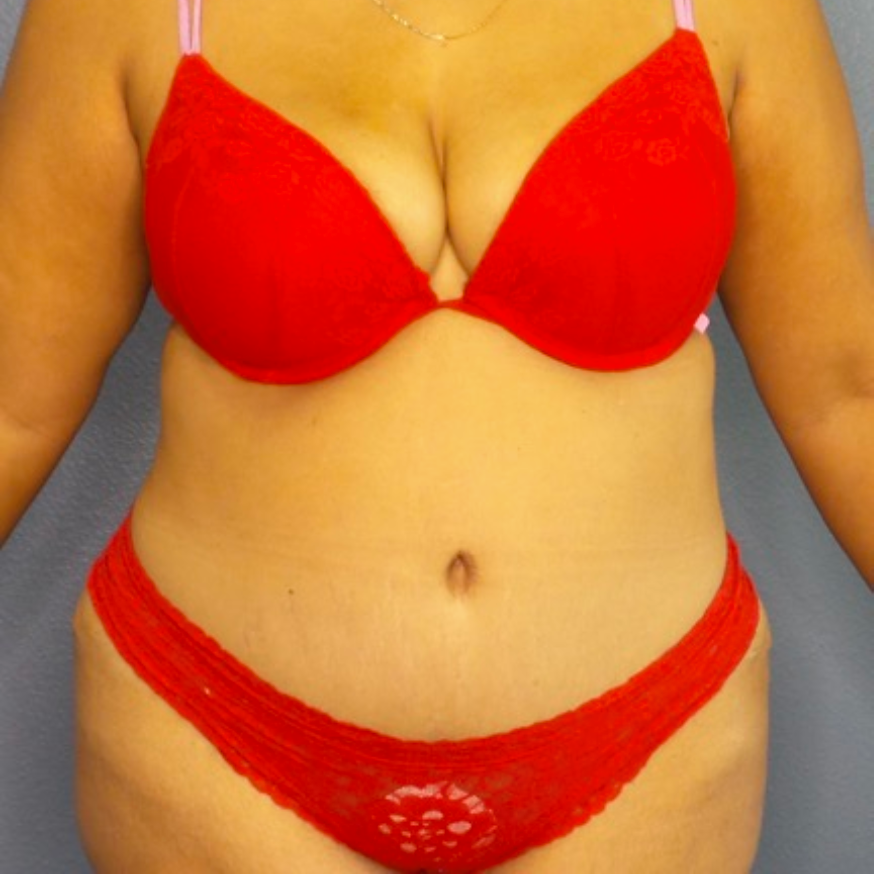
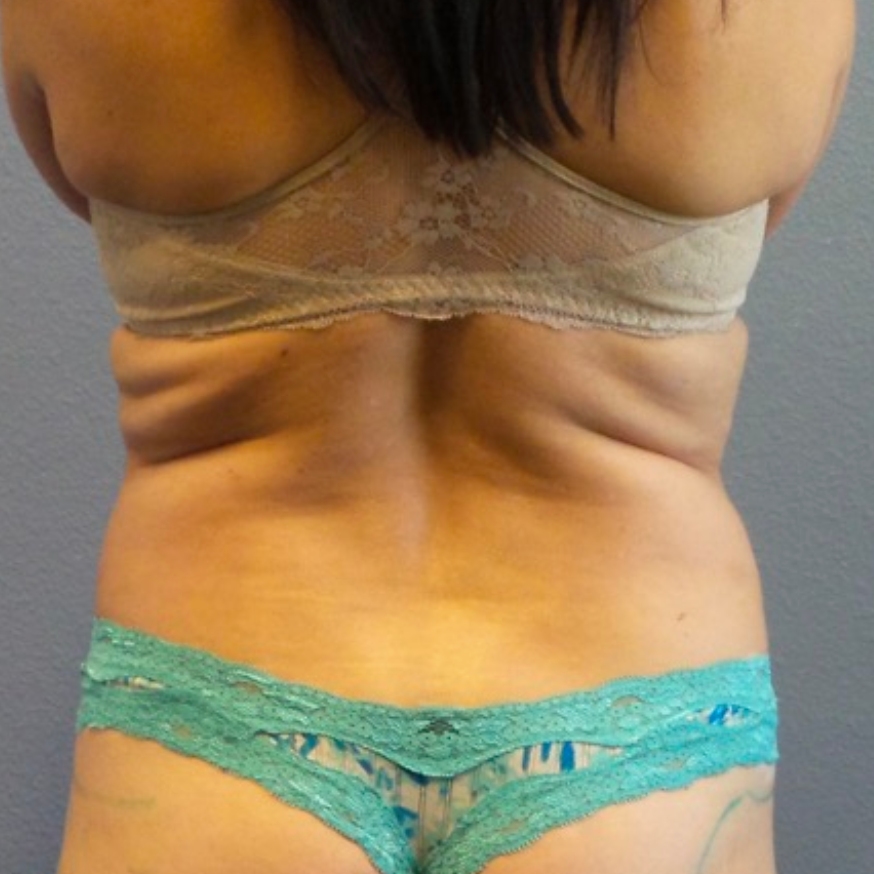
Before and After Photos
Procedure
Breast augmentation is a personal decision, but there are several reasons why women may consider this procedure. Many women experience a loss of breast volume or shape due to pregnancy, breastfeeding, weight fluctuations, or aging. Breast augmentation can help restore the breasts to their pre-pregnancy or youthful appearance. Additionally, breast augmentation can help women with asymmetrical breasts or those who have always desired larger breasts to achieve their desired look.
Before undergoing breast augmentation, women will need to schedule a consultation with a qualified and experienced plastic surgeon to discuss their goals and determine the best approach for their individual needs. During the procedure, the surgeon will make an incision and insert the implant either under the breast tissue or chest muscle. There are several types of implants available, including silicone, saline, and gummy bear implants. Your surgeon will help you choose the best implant for your desired outcome.
Benefits
Breast Enhancement can provide numerous benefits, including an improved self-image, increased confidence, and a more balanced appearance. The procedure can also help to correct asymmetrical breasts, which can make clothes fit better and increase overall satisfaction with one’s appearance.
Expectations
Breast augmentation surgery typically takes about 1-2 hours and is performed under general anesthesia. During the procedure, the surgeon will make an incision either under the breast, around the areola, or in the armpit, and insert the implant. The implant can be placed either under or over the chest muscle.
The type of implant used will depend on several factors, including the patient’s body type, the desired outcome, and the surgeon’s recommendation. There are two main types of breast implants: saline and silicone. Saline implants are filled with sterile saltwater and can be adjusted for size during the surgery. Silicone implants are filled with silicone gel and have a more natural feel.
Risks
Like any surgical procedure, breast enhancement carries some risks and potential complications. These include bleeding, infection, scarring, and implant rupture. Patients may also experience temporary or permanent changes in nipple sensitivity or breast sensation.
Recommended Age
When considering breast augmentation, age becomes an important aspect to consider. While there is no specific age requirement, it is generally recommended that patients wait until they are at least 18 years old before undergoing this procedure. For younger patients, like 22-year-olds, it’s crucial to ensure they have realistic expectations and are mentally and emotionally prepared for the changes that come with breast augmentation.
Choosing a Surgeon
When considering breast enhancement surgery, it’s important to choose a qualified and experienced surgeon who is board-certified in plastic surgery. Patients should also research the type of implant they wish to use and discuss their options with their surgeon to ensure the best possible outcome.
Consultation
Before scheduling the surgery, a thorough consultation with a board-certified plastic surgeon is essential. During this consultation, the surgeon will assess the patient’s overall health, discuss their desired outcome, and evaluate their breast anatomy. They will also go over the different types of implants, incision options, and implant placement techniques to help the patient make an informed decision.
Recovery and Results
Recovery time after breast augmentation varies from patient to patient, but most women can resume normal activities within a few days to a week after the procedure. Patients may experience some swelling, bruising, and discomfort, but this should subside within a few weeks. Results of breast augmentation are immediately visible, but it may take a few weeks for the breasts to settle into their final shape and size. Most women are thrilled with their results and report increased confidence and satisfaction with their appearance.
Cost
The cost of the surgery varies depending on several factors, including the surgeon, location, and type of implant used. The average cost is around $6,500, but it can range from $3,000 to $10,000 or more.
Patients should prepare to pay for breast augmentation surgery out of pocket since insurance typically does not cover it, unless it is part of breast reconstruction surgery following a mastectomy.
Conclusion
Breast augmentation can be a transformative procedure for many women, offering improved self-esteem, a more proportional figure, and a boost in overall quality of life. However, it’s important to fully understand the procedure and its potential risks before making a decision. By choosing a qualified surgeon and following proper aftercare instructions, patients can achieve beautiful and natural-looking results.
FAQ
What are the different types of tuberous breasts?
Type I, (hypoplasia) underdevelopment of the lower inner (medial) fourth (quadrant); type II, (hypoplasia) underdevelopment of the lower inner (medial) fourth (quadrant) and outside (lateral) fourth; and type III, severe breast tightening and narrowing (constriction) and overall underdevelopment (global hypoplasia).Tuberous Breast Deformity: Classification and Treatment Strategy for Improving Consistency in Aesthetic CorrectionKolker, Adam R.; Collins, Meredith S.Plastic & Reconstructive Surgery. 135(1):73-86, January 2015.
What medical conditions have not had safety and effectiveness established in accordance with FDA standards with respect to saline breast implantation?
Safety has not been established in hematologic disorders that interfere with blood clotting, blood thinning, wound healing, immune system derangements, such as immunosuppressive regiments for cancer, HIV, autoimmune conditions like scleroderma, or lupus, and compromised blood supply, as would be found after radiation for cancer.
What is wound dehiscence after breast augmentation surgery, what are the consequences, and what is the treatment?
Wound dehiscence is a disruption of the incision used to access the breast implant pocket in breast enlargement. It may be due to infection, impaired healing, or post-operative trauma. If due to infection, antibiotic therapy or even removal may be necessary as outlined in the question on infection. If due to impaired healing, the precise factor(s) must be identified and addressed, though the breast implant needs may be salvaged. If Caused by trauma, and no breast implant exposure is noted, either primary, or delayed wound closure may be used.
Smooth Vs. Textured
There are advantages and disadvantages to using each type of implant for both the surgeon and the patient. Let’s address the concerns of the patient and then discuss the benefits of one type over another in terms of technical pluses and minuses.A lot of information on the differences in outcomes between using textured or smooth implants for breast augmentation comes from anecdotal reporting or confounded studies and is thus as useful as preference or hearsay. With that in mind here are the supposed advantages of one over another with respect to different characteristics that affect breast enlargement.Textured breast implants have a rough surface. The thinking behind this is that if the texture is irregular, cells that form a scar will not as be organized on such a surface, which would result in a softer, more pliable breast capsule, and diminish contracture rates. This disparity in breast capsule formation has never been conclusively demonstrated in studies. Because the textured breast implant does not have a regular surface, it is thicker, but at the same time thought to be weaker, because of its surface flaws. A well-known consequence of using the textured breast implant is its more tenacious adherence to the breast capsule that is laid down around it. This may be an advantage in a woman who has had a teardrop or anatomical implant placed at the time of breast augmentation to hold position. The reason that is important is that the shaped breast implant is not symmetric and must sit in the subglandular or submuscular pocket just so. In a thinner woman, or a woman with minimal native breast tissue, a breast implant that has adhered to the capsule and is not free to move around, will pull on the adjacent breast skin and cause breast surface irregularities. It will also be more palpable, and it may need to be placed through a larger incision owing to its lack of pliability when compared to a smooth breast implant. Textured breast implants may also carry a higher risk of breast implant rupture because any traction or pushing on the breast would also shear the breast implants by virtue of their close association with the breast pocket capsule. Finally, it may be more difficult to remove textured breast implants, again because of their adherence to the breast capsule.
What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Siltex Contour Profile® Spectrum® Breast Implants?
What are their advantages and disadvantages? The Spectrum® Postoperatively Adjustable Breast Implants are made only by Mentor. The obvious advantage to the adjustability of such breast implants is the ability to enlarge or diminish the size of a breast after the operation. Many women that are quite pleased with the results of their breast augmentation surgery wish that they would have opted for breast implants that were slightly larger, or slightly smaller. Indeed, the decision as to how large of a breast augmentation to request is a tough one. Short of wearing a silicone prosthesis to determine the optimal size for breast enlargement in any particular woman, there are not very many options. This is not what one would call high tech, and at times some patients are unsure in spite of such a maneuver. The Spectrum® Postoperatively Adjustable Breast Implants give a plastic surgeon the ability to adjust the dimensions of an enlarged breast for up to half a year after the fact. This is performed via a tiny fill tube left in place at the time of surgery. The breast implants are filled to a point deemed perfect by the patient, and the tube is removed in the office. Another very powerful reason to use such adjustable implants is when performing breast enlargement surgery along with breast lift surgery. Because the complication rate for this procedure is unacceptable to some aesthetic surgeons they chose to stage it, or perform the breast augmentation separately from the breast lift. The reason for this is that the two procedures stress breast skin more than either one alone. The breast augmentation will stretch breast skin, and place it under tension. The breast lift will excise skin and place it under tension. This excessive tension, in the very least can make for very wide and unattractive scars, and at the worst cause breakdown of the incisions and implant exposure, breast skin and fat (necrosis) death, and possible life-threatening infection. Because the adjustable breast implant does not need to be inflated to full capacity at the time of a simultaneous breast lift and breast enlargement surgery, the augmentation mastopexy can be carried out all in one step. The breast can then be gently and gradually stretched over time. This will obviate the need for an extra surgery. Siltex Contour Profile® Spectrum® Style 2500 breast implant, as the name suggests, is a textured implant of anatomic or teardrop shape, that more accurately reflects the silhouette of a mature breast, with a gentle slope, that can be filled to the desired size as describe above. It is available in the size listed below.

What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Smooth Round Spectrum® Breast Implants? What are their advantages and disadvantages?
The Spectrum® Postoperatively Adjustable Breast Implants are made only by Mentor. The obvious advantage to the adjustability of such breast implants is the ability to enlarge or diminish the size of a breast after the operation. Many women that are quite pleased with the results of their breast augmentation surgery wish that they would have opted for breast implants that were slightly larger, or slightly smaller. Indeed, the decision as to how large of a breast augmentation to request is a tough one. Short of wearing a silicone prosthesis to determine the optimal size for breast enlargement in any particular woman, there are not very many options. This is not what one would call high tech, and at times some patients are unsure in spite of such a maneuver. The Spectrum® Postoperatively Adjustable Breast Implants give a plastic surgeon the ability to adjust the dimensions of an enlarged breast for up to half a year after the fact. This is performed via a tiny fill tube left in place at the time of surgery, adjacent to the incision. The breast implants are filled to a point deemed perfect by the patient, and the tube is removed in the office. The fill volume is generally determined by the amount of injection that a patient would tolerate, and typically that is on order of 50 – 100 cc. Although it is possible to attempt to fill the Spectrum® expandable breast implant years after the surgery in theory, this would probably not be very feasible practically. This is because of the presence of a breast capsule that forms to varying degrees in every breast augmentation patient. Another very powerful reason to use such adjustable implants is when performing breast enlargement surgery along with breast lift surgery. Because the complication rate for this procedure is unacceptable to some aesthetic surgeons they chose to stage it, or perform the breast augmentation separately from the breast lift. The reason for this is that the two procedures stress breast skin more than either one alone. The breast augmentation will stretch breast skin, and place it under tension. The breast lift will excise skin and place it under tension. This excessive tension, in the very least can make for very wide and unattractive scars, and at the worst cause breakdown of the incisions and implant exposure, breast skin and fat (necrosis) death, and possible life-threatening infection. Because the adjustable breast implant does not need to be inflated to full capacity at the time of a simultaneous breast lift and breast enlargement surgery, the augmentation mastopexy can be carried out all in one step. The breast can then be gently and gradually stretched over time. This will obviate the need for an extra surgery. The Smooth Round Spectrum® Style 1400 breast implant, as the name suggests, is a smooth implant of moderate projection that can be filled to the desired size as describe above. It is available in the size listed below.

What are the Spectrum® Postoperatively Adjustable Breast Implants? Particularly the Siltex Round Spectrum® Breast Implants? What are their advantages and disadvantages?
The Spectrum® Postoperatively Adjustable Breast Implants are made only by Mentor. The obvious advantage to the adjustability of such breast implants is the ability to enlarge or diminish the size of a breast after the operation. Many women that are quite pleased with the results of their breast augmentation surgery wish that they would have opted for breast implants that were slightly larger, or slightly smaller. Indeed, the decision as to how large of a breast augmentation to request is a tough one. Short of wearing a silicone prosthesis to determine the optimal size for breast enlargement in any particular woman, there are not very many options. This is not what one would call high tech, and at times some patients are unsure in spite of such a maneuver. The Spectrum® Postoperatively Adjustable Breast Implants give a plastic surgeon the ability to adjust the dimensions of an enlarged breast for up to half a year after the fact. This is performed via a tiny fill tube left in place at the time of surgery. The breast implants are filled to a point deemed perfect by the patient, and the tube is removed in the office. Another very powerful reason to use such adjustable implants is when performing breast enlargement surgery along with breast lift surgery. Because the complication rate for this procedure is unacceptable to some aesthetic surgeons they chose to stage it, or perform the breast augmentation separately from the breast lift. The reason for this is that the two procedures stress breast skin more than either one alone. The breast augmentation will stretch breast skin, and place it under tension. The breast lift will excise skin and place it under tension. This excessive tension, in the very least can make for very wide and unattractive scars, and at the worst cause breakdown of the incisions and implant exposure, breast skin and fat (necrosis) death, and possible life-threatening infection. Because the adjustable breast implant does not need to be inflated to full capacity at the time of a simultaneous breast lift and breast enlargement surgery, the augmentation mastopexy can be carried out all in one step. The breast can then be gently and gradually stretched over time. This will obviate the need for an extra surgery. Siltex® Round Spectrum® Style 2400 breast implant, as the name suggests, is a textured implant of moderate projection that can be filled to the desired size as describe above. It is available in the size listed below.
What is subglandular breast implant placement in breast augmentation?
Subglandular placement refers to placement of breast implants under the skin, fat, and breast tissue, but on top of the muscle. As a result of this, patients who are thin, and lack significant breast tissue will have an increased chance for implant palpability. The risk for capsular contracture is also significantly higher for both saline and silicone breast implants when placed over the muscle. The advantage to using this approach is the ability to take up loose skin at the lower poles of the breasts, and avoid the longer incisions necessary for a breast lift in some cases.
Subglandular Vs. Submuscular Vs. “Dual-Plane” Breast Implant Placement
Subglandular placement refers to placement of breast implants under the skin, fat, and breast tissue, but on top of the muscle. As a result of this, patients who are thin, and lack significant breast tissue will have an increased chance for implant palpability. The risk for capsular contracture is also significantly higher for both saline and silicone breast implants when placed over the muscle. The advantage to using this approach is the ability to take up loose skin at the lower poles of the breasts, and avoid the longer incisions necessary for a breast lift in some cases.Submuscular placement puts the breast implant pocket between the ribs of the chest wall and the chest (pectoralis) muscle on top. It is much less prone to cause breast implant palpability than subglandular implant placement, and is associated with a lower risk of scarring and hardness around the implants. Its disadvantage is the propensity to cause a higher riding breast implant. When the pectoralis muscles are contracted the implants are also prone to move up in a very unnatural fashion.The “dual-plane” approach allows placement of the upper portion of the implant under the muscle, and by releasing the lower portion of the muscle allows the lower portion of the breast implant to sit under the breast tissue. This eliminates the drawbacks of both the sub-glandular and sub-muscular placement while retaining the advantages of both. It is the most commonly performed placement in today’s breast augmentation surgery.
What is submuscular breast implant placement in breast augmentation?
Submuscular placement puts the breast implant pocket between the ribs of the chest wall and the chest (pectoralis) muscle on top. It is much less prone to cause breast implant palpability than subglandular implant placement, and is associated with a lower risk of scarring and hardness around the implants. Its disadvantage is the propensity to cause a higher riding breast implant. When the pectoralis muscles are contracted the implants are also prone to move up in a very unnatural fashion.
What is breast enlargement or breast augmentation surgery?
Breast augmentation or enlargement surgery is an operation in which the breasts are made bigger by the addition of mammary implants. The implants may be saline or silicone. The implant is also known as a mammary prosthesis. It typically takes from one half hour to one and a half hours to place the breast device, depending on such things as pocket location, incision, and the type of implant used.
Should I have breast augmentation using textured or smooth breast implants?
There are advantages and disadvantages to using each type of implant for both the surgeon and the patient. Let’s address the concerns of the patient and then discuss the benefits of one type over another in terms of technical pluses and minuses.A lot of information on the differences in outcomes between using textured or smooth implants for breast augmentation comes from anecdotal reporting or confounded studies and is thus as useful as preference or hearsay. With that in mind here are the supposed advantages of one over another with respect to different characteristics that affect breast enlargement.Textured breast implants have a rough surface. The thinking behind this is that if the texture is irregular, cells that form a scar will not as be organized on such a surface, which would result in a softer, more pliable breast capsule, and diminish contracture rates. This disparity in breast capsule formation has never been conclusively demonstrated in studies. Because the textured breast implant does not have a regular surface, it is thicker, but at the same time thought to be weaker, because of its surface flaws. A well-known consequence of using the textured breast implant is its more tenacious adherence to the breast capsule that is laid down around it. This may be an advantage in a woman who has had a teardrop or anatomical implant placed at the time of breast augmentation to hold position. The reason that is important is that the shaped breast implant is not symmetric and must sit in the subglandular or submuscular pocket just so. In a thinner woman, or a woman with minimal native breast tissue, a breast implant that has adhered to the capsule and is not free to move around, will pull on the adjacent breast skin and cause breast surface irregularities. It will also be more palpable, and it may need to be placed through a larger incision owing to its lack of pliability when compared to a smooth breast implant. Textured breast implants may also carry a higher risk of breast implant rupture because any traction or pushing on the breast would also shear the breast implants by virtue of their close association with the breast pocket capsule. Finally, it may be more difficult to remove textured breast implants, again because of their adherence to the breast capsule.
What are Titanium Coated Breast Implants?
The only difference between silicone impants and titanium coated implants is the presence of titanium coating. This is not a palpable, visible difference. It is a thin layer, and is said to impart more strength to the implant. The inert nature of titanium is also predicted to confer to the coating less of a propensity to cause an inflammatory reaction, and thus diminish the rate of capsular contracture.
Am I too old for breast enlargement or breast augmentation surgery?
“Old” age should never be a determining factor for breast enlargement. Breast augmentation surgery should only be withheld from a patient for two reasons. The first reason would be that the patient is medically unfit to have the surgery. They may have lung, heart, kidney, etc. problems. Operating on such patients would put their life in jeopardy. The second subset of patients should not be operated for psychological reasons. They may have the wrong motivation, they may believe that breast enlargement is the answer to all of their life’s problem, they may a have a skewed perception of their body image, or they may be frankly psychologically unfit to make an informed decision and give consent for breast augmentation surgery. Age itself may slow healing, and require some adjustments in the post-operative period, but provided a patient is healthy, they should not be barred from having their breasts enlarged, simply because they are too old.
Am I too young for breast enlargement or breast augmentation surgery?
Although the media likes to harp on a “disturbing trend,” of high school aged kids getting breast augmentation, this is probably more of a trend in the seedier, non Plastic Surgery Board certified “cosmetic” surgeons. Any responsible plastic surgeon knows full well that results are far more predictable after a breast augmentation performed in a patient who has reached her mature breast size. There are exceptions, of course, the two most common ones are women who have almost no breast tissue to begin with, significant growth is not expected, women with tubular breast deformity, and patient’s with Poland’s syndrome.
What is a transumbilical breast augmentation incision?
The trans-umbilical incision is placed at the top of the belly-button. Its obvious advantage is a lack of scars on, around, or near the breasts. Its disadvantages are blind dissection, making asymmetry more common, and making the likelihood of one side being submuscular and the other subglandular more likely. If an undesirable result is obtained, a new incision will be needed to correct the problem. It may also damage the breast implants, and cannot be used with pre-filled silicone breast implants.
What are Trilucent Breast Implants (Peanut Oil Breast Implants, Soy Oil Breast Implants, Soya Oil Breast Implants, Soybean Oil Breast Implants)?
As the above suggests, such breast implants were filled with various organic oils. The hope was that, as in the case of saline, organic oils would be absorbed in the case of a leak without setting up an inflammatory reaction, in the case of implant rupture. Safety data lacking, along with reports of local inflammatory effects on rupture, the implant was taken off the UK market. Further information from the British MHRA (Equivalent of FDA, except for the food part), can be studied below.Trilucent breast implantsThese implants were removed from the market in 1999, and in 2000 the Medical Devices Agency (now MHRA) recommended that these implants should be explanted.The Trilucent™ Care Centre (TCC), which was set up for patients with these implants, closed on December 31 2004. AIE Inc has taken over the functions carried out by the TCC.A clinical research programme was sponsored by AIE Inc and was carried out by an independent panel of experts to investigate the long-term health effects of Trilucent™ implants. This was completed in 2004. The full report concluded that there is no evidence for local or systemic disease risk once the implants have been removed.MHRA issued a device alert (MDA/2004/047) in September 2004 to notify interested parties of the conclusions of this study and the closure of the TCC.Background informationTrilucent™ breast implants were available for sale throughout the EU between 1995 and March 1999. They consisted of a silicone elastomer shell with a lipid filler based on soyabean oil. Since they were first marketed in 1995 over 9,000 implants were sold in the UK, and implanted into almost 5,000 women.As a result of an investigation into reports of inflammation associated with rupture of Trilucent™ breast implants, MDA reviewed the manufacturer’s safety assessment. This revealed serious concerns relating to the long term safety of Trilucent™ breast implants, in particular in relation to the breakdown of the lipid filler. As a result of MDA’s concerns the company voluntarily withdrew the product from the market in March 1999 and MDA issued an Advice Notice AN 1999(01). The withdrawal was a precautionary measure until further information could be gathered about the biological safety and clinical experience with these implants. The advice given at that time was that there was no evidence to suggest that removal of Trilucent™ breast implants was indicated but that women should be advised to seek an immediate consultation if they noticed unusual breast swelling or inflammation associated with their Trilucent™ breast implants.In AN 1999(01) clinicians were advised not to use MRI (magnetic resonance imaging) on women implanted with Trilucent™ breast implants, because of concerns about heating up of the transponder (a device contained within the implant to provide identification information electronically). In the light of subsequent enquiries on the value of MRI for detecting implant rupture, this advice was modified. Clinicians have since been advised to use MRI with caution on women implanted with Trilucent™ breast implants.The first results of further analytical studies on the filling material in Trilucent™ breast implants became available in May 2000. MDA convened a group of independent experts (the Trilucent™ Advisory Group) to consider the available scientific evidence. This Group made an assessment of the risk to women with these implants and provided advice to MDA. A statement summarising the Trilucent™ Advisory Group’s conclusions and advice is available, with the minutes of a meeting held on 19 May 2000 . Based on this advice, MDA issued a Hazard Notice (HN 2000(05)).In 2000, a programme of research was initiated to investigate any potential risks to women implanted with Trilucent™ breast implants. This programme was directed, on behalf of AEI Inc, by a panel of independent experts. The programme was completed in 2004. The panel concluded that:the recommendation that Trilucent™ breast implants should be removed remains appropriate because exposure of local tissue to toxic compounds has been confirmed;there is no evidence for local or systemic disease risk once the implants have been removed; no further studies are needed to assess the potential risk of Trilucent™ breast implants. This information was the subject of MHRA Medical Device Alert MDA/2004/047.Although the research programme has been completed, MHRA continues to record and investigate reports of adverse events associated with Trilucent™ breast implants. If further problems are identified, MHRA will issue advice.Safety information published by MHRA relating to Trilucent™ breast implants:AN1999(01) – Trilucent breast implants: Voluntary recallVoluntary recall of Trilucent breast implantsHN 2000(05) – Trilucent™ breast implants: recommendation to removeRecommendation to explant Trilucent™ breast implantsMDA/2004/047 – Trilucent (soya bean oil filled) breast implantsConclusions of clinical research programme and closure of TCC
Incision Type
The inframammary approach to breast augmentation surgery allows your plastic surgeon the greatest visibility while dissecting an implant pocket, and as such, allows greater precision and control of symmetry. The inframammary incision is placed in the fold under the breast, in the breast fold, and tends to be more noticeable for two reasons. It is typically larger than other types of breast incisions. It has a tendency to migrate as the implant settles up or down, and its final resting place is less predictable.The periareolar incision goes around the nipple. It is less noticeable, smaller than the inframammary incision, and if a lift is required later in time, it may be utilized again. Its disadvantages in breast enlargement surgery are a higher infection (because the cut goes through breast tissue), it may be more difficult to breastfeed after this type of breast augmentations, and it may decrease breast skin and nipple sensation or infection more of a concern.Placing the incision in the axilla (underarm) allows for a smaller incision. It is useful when there is not much breast tissue present to begin with, and thus no prominent breast fold, and when the nipple-areola complex is small. It is not as well concealed as the periareolar incision, gives less control in terms of the ability to feel around the breast pockets in assuring symmetry, and placement of silicone breast implants will necessitate a larger incision which may be less pleasing.The peri-umbilical incision is placed at the top of the belly-button. Its obvious advantage is a lack of scars on, around, or near the breasts. Its disadvantages are blind dissection, making asymmetry more common, and making the likelihood of one side being submuscular and the other subglandular more likely. If an undesirable result is obtained, a new incision will be needed to correct the problem. It may also damage the breast implants, and cannot be used with pre-filled silicone breast implants.
What is implant exposure or breast implant extrusion after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatment?
The exact risk percentage may be found for both the Allergan, and the Mentor studies in related questions. The problem most often is the result of inadequate soft tissue (breast and skin) overlying the breast implants. It is unusual in cases of primary augmentation, and is more commonly seen in the compromised soft tissue of the reconstructed breast, with or without a history of radiation. If the extrusion if only threatened, and no implant is actually showing at the time of detection, a salvage procedure may be done to improve the soft tissue cover atop the implant. If the implant is exposed, many plastic surgeons will not attempt salvage, as by definition, and exposed implant is a contaminated implant. The breast implant will be removed, patient allowed to heal, and implant replaced at a later time.
Is implant removal common after breast augmentation surgery with silicone breast implants?
Silicone, and for that matter saline breast implants are deemed by both Mentor and Allergan as “not lifetime devices.” The longer the implants have been present the higher the chances for removal with or without replacement for various reasons. Grounds for removal +/- replacement may include displeasure with the cosmetic appearance of the breasts, severe capsular contracture, etc. Silicone breast implant removal and replacement puts the patient at greater risk for complications and reoperations in the future.In Mentor’s Core Study, patients who’d undergone primary breast augmentation, 5% had implant removal at least once over the first 3 post-operative years. In revision-augmentation patients, the rate was 12% over the same time span. In both populations, patient choice and capsular contracture were the primary reasons for removal.In Allergan’s Core Study, patients who’d undergone primary breast augmentation, 9% had implant removal at least once over the first 4 post-operative years. In revision-augmentation patients, the rate was 12% over the same time span. In both populations, patient choice and capsular contracture were the primary reasons for removal.Most patients in who have had their implants explanted, will opt for replacement. Some women do not. This may have adverse cosmetic effects in itself, such such puckering, wrinkling, loose skin, dimpling, and drooping.
Is there a way to tell my own breast tissue from silicone breast implants during self examination?
Distinguishing native breast tissue from silicone breast implants is a necessary part of breast self-examination. It may be more difficult to tell breast tissue from silicone breast implants as compared to saline breast implants given the softer consistency of silicone. If you encounter difficulties, ask your plastic surgeon to demonstrate the appropriate technique for breast self-examination and point out the difference between breast implants and breast tissue.
Is infection common after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatment?
Any surgery, in any discipline carries a risk of infection. The risk is calculated based on the degree of contamination for a particular operation. Breast augmentation is considered a “clean” surgery, and carries an overall infection rate of less than two percent. If infection should take place, it will most often affect one or more of three patterns, assuming there is no disseminated spread, and the infection remains localized. Infection can occur in the skin, in the soft tissue surrounding the implant, and in the form of a pus pocket. Skin infection will usually respond to oral antibiotics. Soft tissue infections surrounding the breast implant may respond to oral antibiotics, will sometimes require intravenous antibiotics, and in other cases need to be treated with implant removal. If the infection should progress to, or start out as an abscess (pus-pocket), the only treatment that will be effective in treating the infection and preventing more serious systemic complications is drainage of pus and breast implant removal. Toxic Shock Syndrome (TSS) may result from the presence of a foreign body (breast implant in this case) in the setting of an infection, and is a truly life-threatening condition that needs to be addressed immediately. It is marked by high fever, nausea and vomiting, diarrhea, light-headedness and possibly loss of consciousness, and a diffuse rash. The treatment is timely institution of IV antibiotics, and breast implant removal. If the breast implant is removed, the infection should be treated, the inflammation allowed to resolve, and a new implant placed weeks down the road.
Should I have breast augmentation using silicone or saline prosthesis?
To begin with let’s address the controversy surrounding silicone filled breast implants. There is a well known reporter of Asian descent married to an ex-talk show host who built a career on sensational reporting without any basis in fact, that cheated millions of women out of a perfectly soft, and natural silicone breast augmentation. As is the case with many such issues, the truth was not nearly as well publicized. For a decade, the truth was not made public at all. To add to this, countless cases of alleged harms stemming from the implantation of silicone implants were exploited by immoral attorneys. Since the 1990’s, silicone breast implants have been shown to impart no increase in the incidence of breast, cancer, immune disease, or any other malady so eagerly imparted to them by dishonest litigators, and melodramatic fortune seekers with no regard for the effect it would have on women interested in breast augmentation, and especially augmentation combined with a breast lift. Silicone implants have several drawbacks but in the opinion of many plastic surgeons, such shortcomings are far outweighed by the benefits afforded by their use in breast augmentation. Silicone breast implants are thought to produce a softer breast, less breast contour deformities, and a substantially more natural feel on breast contact than saline breast implants. The drawbacks to silicone mammary prosthesis use are twofold. The first is difficulty in the detection of breast implant rupture. When silicone breast implants rupture, the saline that was used to fill them is reabsorbed, and the discrepancy between what was and what is, or between what the size of the unaffected breast and the side of the affected breast is very obvious. When silicone breast implants rupture, the silicone fill is not absorbed. The change in the affected breast is more consistent with a shape change than a size change. As a consequence, this becomes much more difficult to detect. This would not be a problem, however, silicone incites a significant inflammatory reaction in many patients, leading to a dense capsule, and making it difficult to remove the old breast implant, and achieve a predictable result in placing the new one at the same operation. Staging, or breaking the operation presents the patient with the nuisance of two surgeries. For this reason, a patient with silicone breast implants must be very vigilant in monitoring for signs of implant rupture, as early detection, and re-implantation, makes it much less likely that a significant inflammatory reaction, or a tough breast implant capsule will form.
Is pain normal after breast augmentation surgery with silicone implants?
The breast enlargement surgery itself should be painless, whether performed under sedation with local anesthetic, or general anesthesia. It is the postoperative period that some may find brings discomfort. Assuming the postoperative course is without complication, pain from breast augmentation surgery peaks the day after surgery, and diminishes over the course of the following three to four days to be tolerable enough without the use of narcotics. Of course, pain tolerance varies significantly from patient to patient. The 72-96 hrs time frame is a “ballpark” figure, and a reflection of personal experience. Several other important factors are crucial to consider in breast enlargement surgery. Pre-incisional administration of local or dilute local (called tumescent) anesthetic greatly diminishes postoperative breast enlargement surgery pain. Postoperative pain and tenderness can be further affected by breast implant placement position. Sub-muscular placement or “dual plane” placement may cause substantially more discomfort than sub-glandular placement because of muscle dissection. If a skin excision is necessary, pain may be more pronounced. The injection of local anesthetic at the conclusion of breast augmentation surgery greatly diminishes postoperative discomfort. In addition, a more effective multiple intercostal nerve block may be performed. Finally, a small catheter may be placed, within the breast implant pocket, for the purpose of delivering local anesthetic in the post breast enlargement surgery period. This has been shown in studies to help significantly with postoperative discomfort. In short, breast enlargement surgery is very well tolerated in most patients.Having stated all of this, it is important to note that pain should get progressively better. Pain that is persistent, uncontrolled by pain medication, or increases in duration and/or intensity after breast augmentation may be a sign of post-operative complication and requires prompt attention.
Is seroma (fluid accumulation as your body attempts to fill a potential space) common after breast enlargement surgery with silicone breast implants, what are the consequences, and what is the treatme
In breast augmentation surgery, a space is created under the soft tissues of the chest wall. This is the space where the breast implant is placed. The size of the pocket and the size of the implant are seldom identical, and the discrepancy creates a potential space where fluid can collect. The dissection through the soft tissue to the breast implant pocket also makes a potential space for fluid to collect. Sometimes a long standing blood collection can leave a space after it is resorbed. Most seromae resolve with only a few drainage attempts, without an incision. In cases where post-breast augmentation seromae fail to resolve, lining of the fluid pocket needs to be excised because this is what makes the fluid.
Is breast tissue or skin flap death (necrosis) common after breast augmentation surgery with silicone breast implants?
Necrosis of breast tissue or soft tissues and skin is very unusual after breast augmentation only, it is much more likely in the case of a breast lift where a significant amount of tissue is removed. Necrosis is the result of loss of blood supply, hence oxygen, leading to tissue death. Blood supply is lost when dissection proceeds through tissue containing vessels that supply a given area, and transects them. Since multiple vessels usually supply any given area of breast and skin, sacrifice of a few vessels is usually well tolerated. The use of nicotine in any form (smoking, chewing, gum, patch, etc.), chemotherapy, radiation, corticosteroids, contamination all compromise blood supply and make the sacrifice of even a few vessels a significant risk for breast tissue death.
Am I a good candidate for the use of silicone gel-filled breast implants in my breast augmentation surgery?
Silicone implants have several drawbacks but in the opinion of many plastic surgeons, such shortcomings are far outweighed by the benefits afforded by their use in breast augmentation. Silicone breast implants are thought to produce a softer breast, less breast contour deformities, and a substantially more natural feel on breast contact than saline breast implants. The drawbacks to silicone mammary prosthesis use are twofold. The first is difficulty in the detection of breast implant rupture. When silicone breast implants rupture, the saline that was used to fill them is reabsorbed, and the discrepancy between what was and what is, or between what the size of the unaffected breast and the side of the affected breast is very obvious. When silicone breast implants rupture, the silicone fill is not absorbed. The change in the affected breast is more consistent with a shape change than a size change. As a consequence, this becomes much more difficult to detect. This would not be a problem, however, silicone incites a significant inflammatory reaction in many patients, leading to a dense capsule, and making it difficult to remove the old breast implant, and achieve a predictable result in placing the new one at the same operation. Staging, or breaking the operation presents the patient with the nuisance of two surgeries. For this reason, a patient with silicone breast implants must be very vigilant in monitoring for signs of implant rupture, as early detection, and re-implantation, makes it much less likely that a significant inflammatory reaction, or a tough breast implant capsule will form. The ideal candidate for a primary breast augmentation with silicone, therefore, is a patient who is secure with silicone as implantation material, is very aware of the shape of her breasts, and vigilant in monitoring for rupture, has a very small amount of native breast tissue, or has a significant amount of droop and desires sub-glandular placement to correct it with minimal incisions.
Is silicone gel-filled breast implant rupture obvious, or easily detected?
When a saline implant ruptures, the saline absorbed within a matter of days. When a silicone breast implant ruptures, the silicone is not absorbed. It remains within the capsule. The shape of the breast may change, but the difference is often undetected in the majority of cases, by patient and by surgeon, and the diagnosis must be established via MRI. If rupture is detected, the breast implant should be removed.In the Allergan Core Study, primary silicone breast augmentation patients who were screened by MRI had a rupture rate of 2.7%, and silicone breast implant revision-augmentation patients had a rupture rate of 4% over 4 years. A European study found a rupture rate of 11% over 15 years. In either study there were no instances of gel migration. There are currently large Allergan post-approval studies being conducted for further safety data.In the Mentor Core Study, primary silicone breast augmentation patients who were screened by MRI had a rupture rate of .5%, and silicone breast implant revision-augmentation patients had a rupture rate of 7.7% over 3 years.
Are unsatisfactory results common after breast augmentation surgery with silicone breast implants?
A patient may be displeased for any number of reasons. The incidence of reoperation for a unsatisfactory result was shown to be roughly 2% of the 15-24% overall re-operative incidence in primary breast augmentation. So, the incidence of reoperations for unsatisfactory results seems to be well below 1%. This is not to say that all patients who are dissatisfied, are dissatisfied to the point of wanting another operation to address the problem, but the answer to whether or not unsatisfactory results are common is no.
Is breast tissue or skin flap death (necrosis) common after breast augmentation surgery?
Necrosis of breast tissue or soft tissues and skin is very unusual after breast augmentation only, it is much more likely in the case of a breast lift where a significant amount of tissue is removed. Necrosis is the result of loss of blood supply, hence oxygen, leading to tissue death. Blood supply is lost when dissection proceeds through tissue containing vessels that supply a given area, and transects them. Since multiple vessels usually supply any given area of breast and skin, sacrifice of a few vessels is usually well tolerated. The use of nicotine in any form (smoking, chewing, gum, patch, etc.), chemotherapy, radiation, corticosteroids, contamination all compromise blood supply and make the sacrifice of even a few vessels a significant risk for breast tissue death.
What Were The Main Reasons For Implant Removal in The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants?

What Were The Complication Rates After 4 Years of Follow-up?
Complications rates reported In The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants are shown below.
*Most events were assessed with severity ratings, and the rates shown in the table include only complications rated moderate, severe or very severe (excludes mild andvery mild ratings). All occurrences of reoperation, implant removal, implant rupture,implant extrusion and pneumothorax are included.**There was one patient with a suspected rupture of one of her implants. This rupture has not yet been confirmed with removal and visual inspection of the implant.Complications rates reported In The Allergan Core Study for Revision Augmentation with INAMED® Silicone Breast Implants are shown below.
Revision-Augmentation: Complications
*Most events were assessed with severity ratings, and the rates shown in the table include only complications rated moderate, severe or very severe (excludes mild andvery mild ratings). All occurrences of reoperation, implant removal, implant rupture,implant extrusion and pneumothorax are included.**There were two patients with a suspected rupture in one of their implants. These two ruptures have not yet been confirmed with removal and visual inspection of the implants.
What Were The Benefits to Silicone Breast Augmentation according to the Allergan Core Study on INAMED® Silicone-Filled Breast Implants?
Positive outcomes in the Allergan Core Study with INAMED® Silicone-Filled Breast Implants were reported as follows.In patients who have undergone primary silicone breast augmentation, 396 (87%) of the total 455 patients underwent breast measurement within 1 and ½ years of surgery. 41% increased by 1 cup size; 45% increased by 2 cup sizes; 8% increased by more than 2 cup sizes; and 6% had no increase or decrease. Patient contentment was based on a 5-point scale evaluation of satisfaction with their silicone breast implants at the time of the follow-up visits. Of the total 455 breast augmentation patients in the study, 364 (80%) gave a satisfaction rating four years after surgery. 346 (95%) were satisfied with their silicone breast implants. According to the commonly used mental and physical health scales (SF-36) and self-esteem scale (Rosenberg), primary augmentation patients showed no significant changes after 4 years. There was a decrease in self-concept on the Tennessee Self Concept Scale in the same time span.Breast measurement were not obtained in revision-augmentation patients since implants were already in place and being exchanged.Patient contentment was based on a 5-point scale evaluation of satisfaction with their silicone breast implants at the time of the follow-up visits. Of the total 146 breast revision-augmentation patients in the study, 111 (76%) gave a satisfaction rating four years after surgery. 96 (87%) were satisfied with their silicone breast implants. According to the commonly used mental and physical health scales (SF-36) and self-esteem scale (Rosenberg), primary augmentation patients showed no significant changes after 4 years. There was a decrease in self-concept on the Tennessee Self Concept Scale in the same time span.
What was the followup rate in the Allergan Core Study on INAMED® Silicone-Filled Breast Implants?
The Allergan Core Study on INAMED® Silicone-Filled Breast Implants has divulged an overall risk for any given complication over the first four years of 41% in primary breast silicone augmentation patients, and 57% for revision-augmentation patients. Of the 455 primary silicone breast augmentation patients enrolled in the study, 83% followed up (378 patients). Of the 147 primary silicone breast augmentation patients enrolled in the study, 83% followed up (378 patients). Of the 147 silicone breast reconstruction patients enrolled in the study, 82% followed up (121 patients).
What Were The Main Reasons For Reoperation in The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants?
The Allergan Core Study for Primary Augmentation with INAMED® Silicone Breast Implants showed an additional 345 procedures performed in 135 reoperations involving 103 primary augmentation patients. 39 of the procedures may be attributed to capsular contracture. The follow-up spans 4 years.
What were the Allergan Core Study Results on INAMED® Silicone-Filled Breast Implants for primary augmentation and revision-augmentation?
The Allergan Core Study is the primary clinical study for INAMED® Silicone-Filled Breast Implants. Individual outcomes cannot be predicted by the study, but expectations, benefits, and chances for a particular negative event may become more clear. The study took place over a 10-year period and was performed to determine the safety and value of the use of silicone breast implants in breast augmentation, breast reconstruction, and breast revision, whether after enlargement or augmentation patients.Silicone breast implant patients were seen weekly for a month, at half a year, and then yearly for 10 years. Safety evaluated through noting the incidence of complications, like infection, capsular contracture, silicone implant rupture, and the need for more surgery. Benefits were ascertained via patient satisfaction and quality of life measures. The Allergan Core Study of INAMED® Silicone-Filled Breast Implants involved 715 patients. Procedures performed included 455 primary silicone breast augmentations, 147 revision-augmentations, 98 breast reconstructions, and 15 revision-reconstructions. Silent rupture was assessed via MRI in 158 primary silicone breast augmentation patients, 50 revision-augmentation patients, 51 primary breast reconstruction patients, and 5 revision-reconstruction patients. The imaging was performed at year after operation, and then at 2 year intervals. It was designed to end at year 9 after surgery, and is updated on a rolling basis. The study has thus far reported on the first four years.
What is the difference between silicone and saline filled breast implants?
The difference between the two types of implants is obvious. Silicone breast prostheses are filled with a silicone, while saline breast implants are filled with saline. The actual breast implant sack, called a breast implant shell, is made of a rubbery silicone elastomer, regardless of whether the implants are silicone, or saline. Apart from certain advantages and disadvantages with respect to the technical outcome of breast augmentation surgery, the basic difference is that silicone implants come filled, while saline implants are filled at the time of surgery. One advantage to saline breast implants that stems from this, is the ability to fill the two breasts with slightly different volumes to correct any small changes in size between the two breasts present before surgery. The answer to the question as to which type of implant to use in breast augmentation surgery has supporters on both the saline breast implant, and the silicone breast implant side. To begin with let’s address the controversy surrounding silicone filled breast implants. There is a well known reporter of Asian descent married to an ex-talk show host who built a career on sensational reporting without any basis in fact, that cheated millions of women out of a perfectly soft, and natural silicone breast augmentation. As is the case with many such issues, the truth was not nearly as well publicized. For a decade, the truth was not made public at all. To add to this, countless cases of alleged harms stemming from the implantation of silicone implants were exploited by immoral attorneys. Since the 1990’s, silicone breast implants have been shown to impart no increase in the incidence of breast, cancer, immune disease, or any other malady so eagerly imparted to them by dishonest litigators, and melodramatic fortune seekers with no regard for the effect it would have on women interested in breast augmentation, and especially augmentation combined with a breast lift. Silicone implants have several drawbacks but in the opinion of many plastic surgeons, such shortcomings are far outweighed by the benefits afforded by their use in breast augmentation. Silicone breast implants are thought to produce a softer breast, less breast contour deformities, and a substantially more natural feel on breast contact than saline breast implants. The drawbacks to silicone mammary prosthesis use are twofold. The first is difficulty in the detection of breast implant rupture. When silicone breast implants rupture, the saline that was used to fill them is reabsorbed, and the discrepancy between what was and what is, or between what the size of the unaffected breast and the side of the affected breast is very obvious. When silicone breast implants rupture, the silicone fill is not absorbed. The change in the affected breast is more consistent with a shape change than a size change. As a consequence, this becomes much more difficult to detect. This would not be a problem, however, silicone incites a significant inflammatory reaction in many patients, leading to a dense capsule, and making it difficult to remove the old breast implant, and achieve a predictable result in placing the new one at the same operation. Staging, or breaking the operation presents the patient with the nuisance of two surgeries. For this reason, a patient with silicone breast implants must be very vigilant in monitoring for signs of implant rupture, as early detection, and re-implantation, makes it much less likely that a significant inflammatory reaction, or a tough breast implant capsule will form.
Will I be able to breast feed after breast enlargement surgery with silicone breast implants?
Breast augmentation surgery should not affect breast feeding. Because breasts gain more projection and substance after enlargement, they are typically easier to hold. Because they protrude more, and are easier to hold, breast feeding becomes much easier. Paradoxically breast feeding is actually less difficult for some women, after breast augmentation surgery. Given all of this, however, there are studies showing that women with breast implants report an inability to feed in up to 2/3’s of the implanted population, compared to 7 in 100 for women without breast implants. It is doubtful, however, that matching for size and age such results would ever hold up.
Is breast tissue atrophy common after breast enlargement surgery with silicone breast implants?
Breast tissue atrophy may be a result of aging, or the pressure exerted by a usually large breast implant in relation to the patient breast and chest wall size. This adverse effect is not very common.
Can calcium collections in the breast after silicone breast implant surgery be mistaken for cancer?
Besides cancer, calcium accumulates in areas of trauma or inflammation such as surgical sites. Although calcium present in and/or around tumors has a distinct configuration, it may be mistaken for a possible malignancy with a resultant recommendation for biopsy and/or extirpative surgery. Such procedures may result in the need for implant removal and replacement, or even breast reconstruction. A baseline mammogram prior to breast augmentation , and radiologic centers experienced in the Eklund technique would likely diminish the chances for this.
Is there an association between breast augmentation surgery with silicone breast implants and cancer, or other serious conditions?
According to multiple studies, there is no association between breast enlargement surgery and breast cancer.The following is an excerpt from a US breast implant manufacturers on the subject.“Breast CancerReports in the medical literature indicate that patients with breast implants are not at a greater risk than those without breast implants for developing breast cancer. Some reports have suggested that breast implants may interfere with or delay breast cancer detection by mammography and/or biopsy; however, other reports in the published medical literature indicate that breast implants neither significantly delay breast cancer detection nor adversely affect cancer survival of women with breast implants.Brain cancerOne recent study has reported an increased incidence of brain cancer in women with breast implants as compared to the general population. The incidence of brain cancer, however, was not significantly increased in women with breast implants when compared to women who had other plastic surgeries. Another recently published review of four large studies in women with cosmetic implants concluded that the evidence does not support an association between brain cancer and breast implants.Respiratory/Lung CancerOne study has reported an increased incidence of respiratory/lung cancer in women with breast implants. Other studies of women in Sweden and Denmark have found that women who get breast implants are more likely to be current smokers than women who get breast reduction surgery or other types of cosmetic surgery. Cervical/vulvar cancer – One study has reported an increased incidence of cervical/vulvar cancer in women with breast implants. The cause of this increase is unknown.Other CancersOne study has reported an increased incidence of stomach cancer and leukemia in women with breast implants compared to the general population. This increase was not significant when compared to women who had other types of plastic surgeries.Neurological Disease, Signs, and SymptomsSome women with breast implants have complained of neurological symptoms (such as difficulties with vision, sensation, muscle strength, walking, balance, thinking or remembering things) or diseases (such as multiple sclerosis), which they believe arerelated to their implants. A scientific expert panel report found that the evidence for a neurological disease or syndrome caused by or associated with breast implants is insufficient or flawed.SuicideIn several studies, a higher incidence of suicide was observed in women with breast implants. The reason for the observed increase is unknown, but it was found that women with breast implants had higher rates of hospital admission due to psychiatriccauses prior to surgery, as compared with women who had breast reduction or in the general population of Danish women.Effects on ChildrenAt this time, it is not known if a small amount of silicone may pass through from the breast implant silicone shell into breast milk during breastfeeding. Although there are no current established methods for accurately detecting silicone levels in breast milk, astudy measuring silicon (one component in silicone) levels did not indicate higher levels in breast milk from women with silicone gelfilled implants when compared to women without implants. In addition, concerns have been raised regarding potential damaging effects on children born to mothers with implants. Two studies in humans have found that the risk of birth defects overall is not increased in children born after breast implant surgery. Although low birth weight was reported in a third study, other factors (for example, lower pre-pregnancy weight) may explain this finding. This author recommended further research on infant health.Potential Health Consequences of Gel BleedSmall quantities of low molecular weight (LMW) silicone compounds, as well as platinum (in zero oxidation state), have been found to diffuse (“bleed”) through an intact implant shell. The evidence is mixed as to whether there are any clinical consequences associated with gel bleed. For instance, studies on implants implanted for a long duration have suggested that such bleed may be a contributing factor in the development of capsular contracture and lymphadenopathy. However, evidence against gel bleed being a significant contributing factor to capsular contracture and other local complications, is provided by the fact that there are similar or lower complication rates for silicone gelfilled breast implants than for saline-filled breast implants. Saline filled breast implants do not contain silicone gel and, therefore, gel bleed is not an issue for those products. Furthermore, toxicology testing has indicated that the silicone material used in the Mentor implants does not cause toxic reactions when large amounts are administered to test animals. It also should be noted that studies reported in the literature have demonstrated that the low concentration of platinum contained in breast implants is in the zero oxidation (most biocompatible) state. In addition, two separate studies sponsored by Mentor have demonstrated that the low concentration of platinum contained in its breast implants is in the zero oxidation (most biocompatible) state. Mentor performed a laboratory test to analyze the silicones and platinum (used in the manufacturing process), which may bleed out of intact implants into the body. Over 99% of the LMW silicones and platinum stayed in the implant. The overall body of available evidence supports tat the extremely low level of gel bleed is of no clinical consequence.”
What is capsular contracture after breast enlargement surgery with silicone breast implants?
A breast implant capsule is the firm, sometimes hard and thick, scar tissue that forms on the inside of the breasts and surrounds the breast implants after breast enlargement surgery. This sphere can contract over time, and squeeze the breast implants (capsular contracture). This would certainly place the breast implants in one position with almost no mobility. The positions of the left and right implants may be distorted with respect to each other, and also in relation to the chest wall. The shape of the breasts may also be made abnormal in any direction. The breasts may also feel unnaturally hard. Finally, significant pain may also be present. The incidence of capsular contracture is thought by many plastic surgeons to be related to blood collection, fluid collection, contamination, or infection at the time of breast enlargement surgery. It is also more common when the breast implants are placed on top rather than behind the pectoralis muscle, and in revision-augmentation. Capsular contracture after breast augmentation is also a risk factor for implant rupture owing to the squeezing of the breast implant by the capsule. This capsular contracture is the most common overall reason for re-operation in breast augmentation patients. It is assigned various grades of severity as noted below, with grades 3 and 4 generally requiring re-operation.Baker Grade I:the breast is normally soft and looks naturalBaker Grade II:the breast is a little firm but looks normalBaker Grade III:the breast is firm and looks abnormalBaker Grade IV:the breast is hard, painful, and looks abnormalSurgical options for the treatment of capsular contracture include releasing the capsule circumferentially, completely excising the capsule, or even the former or latter combined with breast implant replacement. Regardless of the intervention, there is no guarantee against the recurrence of capsular contracture.The Mentor Core Study put the risk of capsular contracture at 8% for the first 3 years after operation for primary augmentation patients. In revision-augmentation the risk was much higher at 19% over the first 3 years.The Allergan Core Study put the risk of capsular contracture at 13% for the first 4 years after operation for primary augmentation patients. In revision-augmentation the risk was much higher at 17% over the first 4 years.
Is chest wall deformity common after breast enlargement surgery with silicone breast implants?
Chest wall deformity may be a result of aging, or the pressure exerted by a usually large breast implant in relation to the patient breast and chest wall size. This adverse effect is not very common.
Is there an asociation between silicone breast implants and connective tissue disease?
Pseudoscientific “studies” reported on cases of connective tissue disease supposedly associated with breast implant use in breast augmentation surgery. No study to date has ever demonstrated this. Many women who went on to develop connective tissue disease after breast augmentation attributed the problem to the implants, but the truth is that the incidence of such patients within the breast enlargement population and the general population is the same.The following is an excerpt from a US breast implant manufacturer on the subject.“Connective Tissue Disease (CTD)Connective tissue diseases include diseases such as lupus, scleroderma, and rheumatoid arthritis. Fibromyalgia is a disorder characterized by chronic pain in the muscles and soft tissues surrounding joints, with tenderness at specific sites in the body. It is often accompanied by fatigue. There have been a number of published epidemiological studies which have looked at whether having a breast implant is associated with having a typical or defined connective tissue disease. The study size needed to conclusively rule out a smaller risk of connective tissue disease among women with silicone gel-filled breast implants would need to be very large. The published studies taken together show that breast implants are not significantly associated with a risk of developing a typical or defined connective tissue disease. These studies do not distinguish between women with intact and ruptured implants. Only one study evaluated specific connective tissue disease diagnoses and symptoms in women with silent ruptured versus intact implants, but it was too small to rule out a small risk.CTD Signs and SymptomsLiterature reports have also been made associating silicone breast implants with various rheumatological signs and symptoms such as fatigue, exhaustion, joint pain and swelling, muscle pain and cramping, tingling, numbness, weakness, and skin rashes. Scientific expert panels and literature reports have found no evidence of a consistent pattern of signs and symptoms in women with silicone breast implants. Having these rheumatological signs and symptoms does not necessarily mean you have a connective tissue disease; however, you should be aware that you may experience these signs and symptoms after undergoing breast implantation. If you notice an increase in these signs or symptoms, you should consider seeing a rheumatologist to determine whether these signs or symptoms are due to a connective tissue disorder or autoimmune disease.”
Does baby get exposed to silicone that diffuses from silicone breast implants?
Silicon concentrations in breast milk were not shown to be higher in women with silicone breast implants versus women without silicone breast implants. Silicon is a component in silicone, so that the study looking at this was not wholly conclusive, and if any component of silicone is in fact present in breast milk the effects of this will not be known until further studies are performed.
Policies vary among individual health insurance providers, but there is a chance your health insurance premiums may increase as a result of breast enlargement surgery. Coverage of post-surgical complications also varies substantially. You should check with your insurance company prior to your breast enlargement surgery. It may be wise to purchase a one time coverage for possible complications for a relatively low one time payment.
What is a periareolar incision in breast enlargement surgery?
The periareolar incision goes around the nipple. It is less noticeable, smaller than the inframammary incision, and if a lift is required later in time, it may be utilized again. Its disadvantages in breast enlargement surgery are a higher infection rate (because the cut goes through breast tissue), possible difficulty in breastfeeding, and the risk of decreased sensation in breast skin and nipple sensation.
What are PIP Saline Breast Implants?
(PIP=Poly Implant Prosthesis) Saline Breast Implants are simply silicone elastomer shell breast implants pre-filled with saline. The touted advantages of this breast implant is two-fold. The first is that the implant shell is thinner and, has a more natural feel, like that of silicone implants. The second is that no filling is required at the time of surgery minimizing the risk of contamination, and cutting down operative time. Bot points are moot since the PIP Saline Breast Implants are not available in the US, secondary to a lack of submitted safety data.
Should I opt for Subglandular, Submuscular, or the “Dual-Plane” Breast Implant Placement?
Subglandular placement refers to placement of breast implants under the skin, fat, and breast tissue, but on top of the muscle. As a result of this, patients who are thin, and lack significant breast tissue will have an increased chance for implant palpability. The risk for capsular contracture is also significantly higher for both saline and silicone breast implants when placed over the muscle. The advantage to using this approach is the ability to take up loose skin at the lower poles of the breasts, and avoid the longer incisions necessary for a breast lift in some cases.Submuscular placement puts the breast implant pocket between the ribs of the chest wall and the chest (pectoralis) muscle on top. It is much less prone to cause breast implant palpability than subglandular implant placement, and is associated with a lower risk of scarring and hardness around the implants. Its disadvantage is the propensity to cause a higher riding breast implant. When the pectoralis muscles are contracted the implants are also prone to move up in a very unnatural fashion.The “dual-plane” approach allows placement of the upper portion of the implant under the muscle, and by releasing the lower portion of the muscle allows the lower portion of the breast implant to sit under the breast tissue. This eliminates the drawbacks of both the sub-glandular and sub-muscular placement while retaining the advantages of both. It is the most commonly performed placement in today’s breast augmentation surgery.
What are Polyurethane Coated Breast Implants?
Polyurethane Coated Breast Implants are not available in the U.S. at this time. They were taken off the UK market in 1991, but brought back in 2005 after “The Committee on Carcinogenicity” deemed the carcinogenic risk “small and unquantifiable.” The advantage of the polyurethane implant is thought to be less capsular contracture. A lot of the more seasoned plastic surgeons swear by this type of implant. Further information from the British MHRA (Equivalent of FDA, except for the food part), can be studied below.“Polyurethane-coated breast implants”Polyurethane-coated silicone gel breast implants are now available for implantation in the UK.These implants consist of a silicone elastomer shell filled with silicone gel. The shell is coated with a polyurethane foam which breaks down over time. Polyurethane-coated breast implants were removed from the UK market in 1991, following concerns about the possible carcinogenic risk from the polyurethane breakdown product. The Committee on Carcinogenicity1 concluded that carcinogenic risk from these implants is small and unquantifiable. In April 2005, the manufacturer of one type of polyurethane-coated breast implant reintroduced them to the UK. The manufacturer claims that the incidence of capsular contracture is lower than with other types of breast implant, and that the movement or rotation is less.”
How did MHRA bring the Polyurethane Coated Breast Implant issue to plastic surgery patients?
Information for women considering polyurethane-coated breast implants…What are polyurethane-coated breast implants?Micro-Polyurethane Surfaced (MPS) mammary implants, manufactured by PolytechSilimed Europe GmbH, consist of a silicone elastomer shell, filled with silicone gel.This design is similar to other silicone gel breast implants, except that the shell iscoated with a polyurethane foam, intended to reduce the rate of capsular contracture.What is capsular contracture?Fibrous tissue forms around any implant as part of the body’s response to the implant material. The formation of a fibrous capsule around a breast implant is thus a normal reaction. In some women, however, the fibrous capsule can contract and ‘squeeze’ the implant resulting in an altered appearance and consistency of the breast, and is sometimes associated with pain. This is known as capsular contracture. The amount of contracture varies from person to person and cannot be predicted before implantation. An additional operation to remove the fibrous capsule, and possibly the implant, is sometimes necessary.What are the advantages of polyurethane-coated breast implants?The manufacturer claims that the incidence of capsular contracture is lower for MPS implants than for other silicone breast implants with smooth or textured shells. The texture of the foam coating is believed to disrupt the organisation of the cells that form the fibrous capsule, thus making the capsule less likely to contract. They also claim that movement or rotation of the implant is less likely due to better adhesion to the underlying tissue.What are the risks associated with polyurethane-coated breast implants?Following implantation, the polyurethane foam coating breaks down over several years. After this time, it is thought that the protective effect against capsular contracture may be lost or diminished. One of the chemicals that is released into the surrounding tissue during the breakdown of the coating is known to cause cancer in animal experiments. The risk of developing cancer in humans due to the presence of these implants is small and unquantifiable.How do I decide whether to have polyurethane-coated breast implants?Many factors need to be taken into account when deciding which type of implant is most suitable for a particular person, and the relative importance of these factors varies with individual circumstances. Your plastic surgeon will be able to discuss the options available to you and the advantages and drawbacks of each, so that, between you, you can reach a decision on which type of breast implant would be the most appropriate for you.This information sheet has been produced by the Devices Division of the Medicines and Healthcare products Regulatory Agency (MHRA), which was formerly the Medical Devices Agency (MDA). MHRA is an executive agency of the UK Department of Health whose role is to protect and promote public health and patient safety by ensuring that medicines, healthcare products and medical equipment meet appropriate standards of safety, quality, performance and effectiveness, and are used safely.
How did MHRA bring the Polyurethane Coated Breast Implant issue to plastic surgery professionals?
The following letter was issued by MHRA.“12th April 2005Dear…Reintroduction of polyurethane-coated breast implants in the UKI am writing to you about the decision by Polytech Silimed Europe GmbH, to supply their Micro-Polyurethane Surfaced (MPS) mammary implants for use in the UK, as from April 2005. Polyurethane-coated implants have not been available in the UK since 1991. The purpose of this letter is to bring to the notice of plastic surgeons the risks and claimed benefits associated with these implants, so that surgeons can reach an informed judgement on the suitability of the implants and be able to give appropriate advice to women considering their implantation.BackgroundSilicone gel filled breast implants covered with polyurethane foam coating were introduced to clinical use in the 1970s with the aim of reducing the rate of capsular contracture. They were withdrawn worldwide in 1991 following concern that the polyurethane coating might release a carcinogenic breakdown product. One such breast implant (the MPS implant) was subsequently reintroduced in Europe and the Medical Devices Agency (MDA, now MHRA) issued two Advisory Notices1 to draw attention to the carcinogenic risk and to advise surgeons that these implants should not be used in the UK.Evidence of RiskThe carcinogenic risk arising from polyurethane-coated breast implants was assessed by the Committee on Carcinogenicity2 (COC) in 1991 and 1994. The COC concluded that the implants give rise to a small, unquantifiable carcinogenic risk because the breakdown of the polyurethane coating over a number of years leads to the release of small amounts of the probable genotoxic carcinogen, 2,4-toluenediamine (2,4-TDA). No evidence has emerged since 1994 that would alter the COC’s conclusions. In 2001, MDA prepared a report on the safety of polyurethane-coated breast implants. This report presented the opinion of the COC and discussed factors relevant to the carcinogenic risk assessment. It noted that there were reports of a reduction in capsular contracture with polyurethane-coated breast implants but insufficient evidence was available at that time to demonstrate the long-term benefits of these devices over other products.Further DevelopmentsIn 2003, in response to the MDA report, Polytech Silimed provided MHRA with a review of evidence for a lower, quantifiable rate of capsular contracture with polyurethane-coated breast implants. The manufacturer claimed that the clinical benefits of these implants therefore outweighed the potential risks. In November 2003, the above reports were reviewed by the Committee on the Safety of Devices3 (CSD). The CSD concluded that, on the basis of the evidence available at that time, the benefits were not substantial and did not outweigh the remote but unquantifiable carcinogenic risk. They therefore could not recommend the re-introduction of polyurethane-coated breast implants into clinical use in the UK. In 2004 the manufacturer provided additional evidence which they claimed further supported the clinical benefit of these implants. In January 2005, the manufacturer informed MHRA of their intention to supply MPS mammary implants in the UK. As the implants are CE marked medical devices, the MHRA accepts that they can legitimately be placed on the UK market, provided users and potential recipients are appropriately informed about their risks and benefits. MHRA has placed details of the CSD discussion and a copy of its 2001 report on the safety of these implant on its website for the information of plastic surgeons 4. Information on the benefits claimed for these implants can be obtained from the manufacturer5. Plastic surgeons may also find the attached information sheet useful when discussing the suitability of these implants with their patients.Yours sincerely,Dr Susanne LudgateBSc(Hons) MB ChMB DMRT FRCR FRACRClinical Director (Devices)Medicines and Healthcare products Regulatory Agency (MHRA)”
What can I expect in the post-operative period following breast enlargement surgery?
The following information is given to Dr. Gerzenshtein’s patients before surgery to inform them of a typical course after breast augmentation. Your plastic surgeon may have a different working environment, and his or her patients a different experience. Consult with your physician about their impressions of patient experience. On waking from anesthesia, you will find yourself in the recovery room with dressings and/or bra in place. You will be able to depart once sufficiently recovered from anesthesia. A friend or family member should drive you home and stay with you for the next 2 days to help you with activities of daily living. You will feel tired and run down for the first several days after general anesthesia, this will improve substantially over the first week. Discharge should be minimal over the next 48 hours; bleeding may occur with excessive activity. If dilute local solution was used (superwet or tumescent technique) pain and discomfort will be mild initially, it will increase and peak within two days, it will then subside over the course of one to two weeks, please use pain medication as needed to help. Nausea and vomiting in the postoperative period is not uncommon and has to do with the type of anesthesia used, and overall patient sensitivity to the various medications, it generally resolves within one to two days after surgery, increasing fluid intake, especially via one of the “ade” (gatorade, powerade, etc.) solutions available for sports use, combined with anti-emetic medication should minimize this problem. Use of opiate pain medication, combined with inactivity, and dehydration may lead to constipation, increasing fluid intake will help this as well, especially in combination with walking, and the use of the prescribed stool softener. Swelling and bruising peak within three days of surgery and gradually subside over the following week. Healing incisions will adopt a pinkish hue which should gradually fade over the next six months to a year. Some patients react to absorbable (inside) suture, small pustules or whiteheads along the incision may signal this, the suture may be removed in the office if the problems becomes bothersome. Numbness may affect the breast skin, and/or the nipple, most commonly this involves the lower pole of the breast skin, and resolves on its own within six months.
Are there any particular questions you should ask of your plastic surgeon in consultation?
Any of the questions within this FAQ section may be asked, however because of time constraints, it is best to ask questions that focus on the negatives, possible complications, and possible re-operations.
What is the rate of re-operative breast augmentation surgery?
What are the reasons for early re-operative breast enlargement surgery?
What are the reasons for late re-operative breast enlargement surgery?
Will I ever need another breast operation?
What are the pros and cons of silicone versus saline breast implants?
Why are there all kinds of different shapes, sizes and textures for implants, which one is right for me?
Will the position of my implants change over time?
Will I ever need a breast lift?
If I decide to have the breast implants removed, and not replaced, will my breasts look deformed?
Will I be able to breast feed?
Will the shape and size of my breasts change during, or after pregnancy?
How does the look of implanted breasts change over time?
What happens if I don’t like the way my breasts look?
Asking about a surgeon’s credentials and experience should not make one apprehensive. Allowing a surgeon to alter your body is very personal. You must be very comfortable with the surgeon’s credentials, and with his or her understanding of your expectations.
Can I see some preoperative and postoperative photographs of your work?
Are you board certified in plastic surgery?
Do you do many breast augmentations?
What is your “re-do” percentage?
What is the most common problem you’ve encountered post-operatively?
You should have at least a general idea of what you expect your breasts to look like after augmentation. After all, if you don’t know, how can you expect your plastic surgeon to get a result you will be happy with. Things to consider include breast implant size, and the particulars of shape such as cleavage, upper breast fullness or slope, and implant projection, or perkiness, placement under the muscle, or over it, incision site, and smooth versus textured implants. You should also consider the advantages and disadvantages of silicone breast implants vs. saline breast implants. All of this information will aid your surgeon in choosing the right implant size and type for you.
If I need revision breast augmentation surgery, or “touch up” breast enlargement surgery do I have to pay?
Generally, surgeon fee should be waived for breast augmentation revision surgery. That would leave the cost of the facility fee, supplies, and possibly anesthesia to worry about, which should run a fraction of the cost of the original surgery. A plastic surgeon will typically gladly correct mistakes associated with positioning, size discrepancy, and obvious asymmetry, as this clearly reflects on the surgeon’s ability, but also integrity. Some surgeons will do the same regardless of whether the mistake was their own, or due to patient compliance, accident, etc. The time frame for something like this is typically one year. All conditions must be discussed, and agreed upon by the patient and surgeon; so that there are no misunderstandings should a repeat surgery be necessary. Both implant companies based in the U.S. have extended warranties on both their breast implants, and repeat breast augmentation surgery for a very small fee. This breast augmentation insurance should be seriously considered by anyone undergoing breast enlargement surgery.
What Were The Main Reasons For Implant Removal in The Allergan Core Study for Revision-Augmentation with INAMED® Silicone Breast Implants?
The Allergan Core Study for Revision-Augmentation with INAMED® Silicone Breast Implants showed 32 implant removals performed in 18 prevision-augmentation patients. 26 of the 32 implants were replaced. 9 of the procedures may be attributed to capsular contracture – the most common reason for removal. The follow-up spans 4 years.
Revision-Augmentation: Main Reason For Implant Removal Through 4 Years
What Were The Main Reasons For Reoperation in The Allergan Core Study for Revision-Augmentation with INAMED® Silicone Breast Implants?
The Allergan Core Study for Revision-Augmentation Augmentation with INAMED® Silicone Breast Implants showed an additional 181 surgeries performed in 83 reoperations involving 49 revision-augmentation patients. 14 of the procedures may be attributed to capsular contracture. The follow-up spans 4 years.
Saline Vs. Silicone
To begin with let’s address the controversy surrounding silicone filled breast implants. There is a well known reporter of Asian descent married to an ex-talk show host who built a career on sensational reporting without any basis in fact, that cheated millions of women out of a perfectly soft, and natural silicone breast augmentation. As is the case with many such issues, the truth was not nearly as well publicized. For a decade, the truth was not made public at all. To add to this, countless cases of alleged harms stemming from the implantation of silicone implants were exploited by immoral attorneys. Since the 1990’s, silicone breast implants have been shown to impart no increase in the incidence of breast, cancer, immune disease, or any other malady so eagerly imparted to them by dishonest litigators, and melodramatic fortune seekers with no regard for the effect it would have on women interested in breast augmentation, and especially augmentation combined with a breast lift. Silicone implants have several drawbacks but in the opinion of many plastic surgeons, such shortcomings are far outweighed by the benefits afforded by their use in breast augmentation. Silicone breast implants are thought to produce a softer breast, less breast contour deformities, and a substantially more natural feel on breast contact than saline breast implants. The drawbacks to silicone mammary prosthesis use are twofold. The first is difficulty in the detection of breast implant rupture. When silicone breast implants rupture, the saline that was used to fill them is reabsorbed, and the discrepancy between what was and what is, or between what the size of the unaffected breast and the side of the affected breast is very obvious. When silicone breast implants rupture, the silicone fill is not absorbed. The change in the affected breast is more consistent with a shape change than a size change. As a consequence, this becomes much more difficult to detect. This would not be a problem, however, silicone incites a significant inflammatory reaction in many patients, leading to a dense capsule, and making it difficult to remove the old breast implant, and achieve a predictable result in placing the new one at the same operation. Staging, or breaking the operation presents the patient with the nuisance of two surgeries. For this reason, a patient with silicone breast implants must be very vigilant in monitoring for signs of implant rupture, as early detection, and re-implantation, makes it much less likely that a significant inflammatory reaction, or a tough breast implant capsule will form.
Should I perform self breast examinations after breast enlargement surgery?
Self breast examinations are very important regardless of the presence of breast implants. Imaging studies combined with breast self-examinations may help discover a cancer in its early stages, thereby making treatment more effective.
How can breast implants change the shape of my breasts?
Breast implants are designed to increase the size of the breasts into which they are implanted. The way in which breast shape changes is dictated by the type and size of breast implants used. Wider implants add cleavage, while narrower implants of the same volume increase the projection, or perkiness of the operated breasts. Anatomically shaped, otherwise referred to as contoured, or teardrop breast implants, produce more upper pole fullness, but less of the other characteristics, given that that at the same volume, more fill sits higher in the breast. Using larger breast implants may obviate the need for a mastopexy, or a breast lift, because the larger volume may be able to adequately take up the extra skin present in the cases of significant breast sagging or drooping.
Shaped Vs. Round
This decision depends as much on what the surgeon can accomplish using either the round or the anatomically contoured implants as on which type of implants to use. Assuming the breast pockets are dissected in to the inferior or lowermost extent in the exact same manner, the shaped implants will impart more fullness at the top, and provide a more natural, gently curving slope to the augmented breast. For this to happen, however, the lowermost portion of the dissection or pocket must be the same no matter which mammary implants you use. If a surgeon habitually dissects low enough inferiorly to drop round breast prosthesis low enough that upper pole fullness is lost, that surgeon will likely favor the teardrop shaped, or anatomically shaped implant to compensate for that dissection. For anatomically shaped or contoured implants to do what they were intended for, pocket dissection has to extremely precise. This is because contoured breast implants are not symmetric; they have a top and bottom. If the subglandular or submuscular pockets are too wide, the shaped implants can shift or even flip, imparting asymmetry and even worse an unnatural shape to the augmented breast. Even though shaped mammary implants are textured, this is still no guarantee against malposition. Finally using the round type of breast implants can impart more medial or inner breast fullness. This significantly improves cleavage, because more fill volume winds up toward the more central part of the breast.There are several advantages to the use of high profile breast implants. First, just as the name suggests, for any given fill volume, high profile breast implants will implant more projection or profile when compared to moderate or “normal” breast prosthesis, and most certainly more than low profile, or anatomically shaped breast implants. The way to picture this is that if you had a cone with a highly sitting tip versus a cone with a wide base, the narrower, taller cone would point more (think of Madonna’s show bra!) and thus give more projection to the breast. What this means is that women with a narrower, smaller chest wall can still have larger breasts. The advantage to some women, and disadvantage to others comes from a basic difference in perception as what a natural breast should look like. If a patient prefers a highly “perky,” high profile, or well projecting breast, the high profile breast implant would be considered ideal. High profile breast implants would not be ideal to place in a patient who prefers natural, gently sloping, and slightly ptotic (hanging) breasts, or in a client who is large and wide chested. Placing full profile breast implants in the case of a wide chest would impart a very unnatural “double cone” appearance. For women with a mid-range chest-wall diameter the choice is one of partiality. That is, the decision has to be made between projection, and cleavage. This is because lower profile implants with a wide base will naturally fill up the inner, otherwise known as the medial breast, and produce cleavage. Finally, it is mostly the anecdotal opinion of some authorities that full or high profile implants tend to generate less rippling.
What are MemoryGel™ Smooth Round Moderate Profile Breast Implants? What are their advantages and disadvantages?
As the name suggests the MemoryGel™ Smooth Round Moderate Profile Breast Implants are round in shape (not anatomically shaped), have a moderate profile (not too low, and not too perky), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round moderate profile breast implants is a compromise between upper pole fullness, cleavage, and projection. It is a sort of “jack-of-all trades, master of none.” Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. In addition, the use of silicone allows for the use of the MemoryGel™ Smooth Round Moderate Profile Breast Implants in breasts with less skin and soft tissue cover. Some physicians prefer to use the larger size breast implants in moderate breast ptosis (sagging breasts) to avoid extra incisions. Use of silicone implants, because of their consistency, is ideal in such cases, especially when the lower pole of the breast has little soft tissue cover. The particular proprietary polymer employed in the Mentor devices is a cohesive gel that is purported to have more consistency such that even in cases of ruptured breast implants it is said not to leak as fast or as much. The obvious disadvantage to the moderate round breast implants is that if one particular area of concern needs special attention, a more specialized implant will give better improvement than this all-purpose breast implant.
Smooth Round Gel Moderate Profile Sizing Chart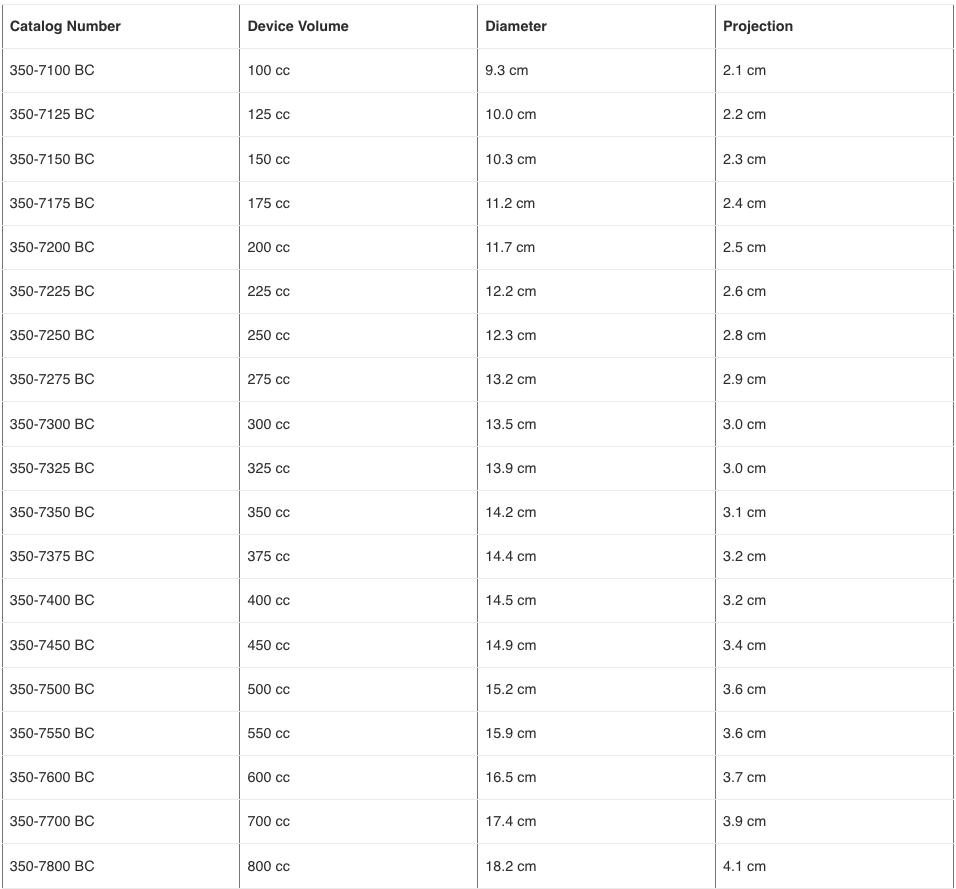
What are MemoryGel™ Smooth Round Moderate Plus Profile Breast Implants? What are their advantages and disadvantages?
As the name suggests the MemoryGel™ Smooth Round Moderate Plus Profile Breast Implants are round in shape (not anatomically shaped), have a moderate plus profile (not too low, but more perky), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round moderate plus profile breast implants is a compromise between upper pole fullness, cleavage, and projection. It is a sort of “jack-of-all trades, master of none.” Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. In addition, the use of silicone allows for the use of the MemoryGel™ Smooth Round Moderate Profile Breast Implants in breasts with less skin and soft tissue cover. Some physicians prefer to use the larger size breast implants in moderate breast ptosis (sagging breasts) to avoid extra incisions. Use of silicone implants, because of their consistency, is ideal in such cases, especially when the lower pole of the breast has little soft tissue cover. The particular proprietary polymer employed in the Mentor devices is a cohesive gel that is purported to have more consistency such that even in cases of ruptured breast implants it is said not to leak as fast or as much. Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. The Plus Profile Breast Implants does, however add a bit more projection, or the protuberance noted on side view at a slight expense to the width of the breast, and thus the cleavage. The obvious disadvantage to the moderate plus round breast implants is that if one particular area of concern needs special attention, a more specialized implant will give better improvement than an all-purpose breast implant.
Smooth Round Gel Moderate Plus Profile Sizing Chart
What are Contour Profile® High Breast Implants Style 2900? What are their advantages and disadvantages?
As the name suggests the Contour Profile® Moderate Breast Implants are contoured to resemble the slope of a natural breast (a subject of debate), have a high (perky) profile, and are encased in a textured silicone elastomer shell, to aid in maintaining their position. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The use of a textured in shell in an anatomic or teardrop implant is absolute. This is because textured implants adhere more intimately to the implant capsule, and have less of a tendency to move. When placing shaped breast implants it is very important to achieve correct positioning, as there is a definite orientation to the implants, hence textured shells are used with contoured breast implants exclusively. The biggest advantage of the contoured breast implants is their ability to add more volume to the upper pole of the breast, and to provide a gentler slope to the breast silhouette. Cleavage is more affected in the high profile implant, as its base is narrower, and the inside part of the breast does not extend as far inward. Projection is improved through a taller implant with a narrower base. The obvious disadvantages to the high profile contoured implants are decreased cleavage, as explained above, and the danger of implants malposition, or flipping within the pocket of dissection. Although at times a turned-about breast implant may be restored to “normal” position without surgery, sometimes a trip to the operating room becomes necessary.
Contour Profile®, High Profile Style 2700 Sizing Chart
What are Contour Profile® Moderate Breast Implants Style 2900? What are their advantages and disadvantages?
As the name suggests the Contour Profile® Moderate Breast Implants are contoured to resemble the slope of a natural breast (a subject of debate), have a moderate profile (not too low, and not too perky), and are encased in a textured silicone elastomer shell, to aid in maintaining their position. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The use of a textured in shell in an anatomic or teardrop implant is absolute. This is because textured implants adhere more intimately to the implant capsule, and have less of a tendency to move. When placing shaped breast implants it is very important to achieve correct positioning, as there is a definite orientation to the implants, hence textured shells are used with contoured breast implants exclusively. The biggest advantage of the contoured breast implants is their ability to add more volume to the upper pole of the breast, and to provide a gentler slope to the breast silhouette. Cleavage is less affected than projection, as breast implant width is maintained, but projection is diminished. The obvious disadvantages to the contoured implants are decreased projection, as explained above, and the danger of implant malposition, or flipping within the pocket of dissection. Although at times a turned-about breast implant may be restored to “normal” position without surgery, sometimes a trip to the operating room becomes necessary.
Contour Profile® Moderate Breast Implants Style 2900 Sizing Chart
What are the various silicone implants available from Mentor?

What are Siltex® Round Moderate Profile Breast Implant Style 2600? What are their advantages and disadvantages?
As the name suggests the Round Saline Breast Implants are round in shape (not anatomically shaped), have a moderate profile (not too low, and not too perky), and are encased in a textured silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round moderate profile breast implants is a compromise between upper pole fullness, cleavage, and projection. It is a sort of “jack-of-all trades, master of none.” Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. The obvious disadvantage is that if one particular area of concern needs special attention, a more specialized implant will give better improvement than this all-purpose breast implant.
Siltex® Round, Moderate Profile Style 2600 Sizing Chart
What are Round Saline Breast Implants? Particularly the Smooth Round High Profile Breast Implants, Style 3000 from Mentor? What are their advantages and disadvantages?
As the name suggests the Round Saline Breast Implants are round in shape (not anatomically shaped), have a high profile, giving them more of a bulge on side view (the most perkiness for your buck), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round high profile implants is this protuberance, considered youthful by many patients, and plastic surgeons. Another considerable advantage is the ability to use higher volume implants in patients with narrow chest walls because the width of the implant base is narrower. The disadvantages include a sacrifice of cleavage, owing to the decrease in the base diameter of the implant, and a drop in upper pole fullness for the same reason. In addition some women find the look imparted by the high profile breast implant too perky.
Smooth Round, High Profile, Style 3000 Sizing Chart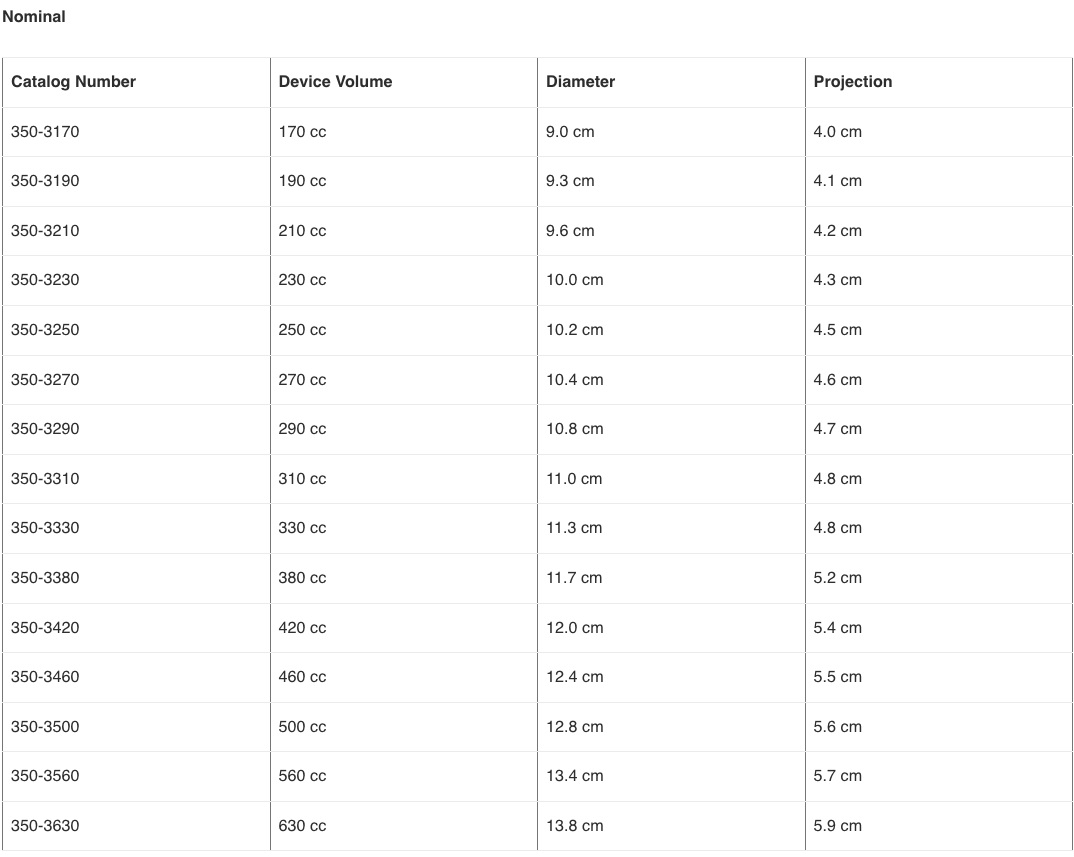


Please Note: Individual implant dimensions may vary slightly in products of this type. Not all units will conform exactly to the dimensions noted above.
What are Round Saline Breast Implants? Particularly the Smooth Round Moderate Profile Breast Implants, Style 1600 from Mentor? What are their advantages and disadvantages?
As the name suggests the Round Saline Breast Implants are round in shape (not anatomically shaped), have a moderate profile (not too low, and not too perky), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round moderate profile breast implants is a compromise between upper pole fullness, cleavage, and projection. It is a sort of “jack-of-all trades, master of none.” Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. It is touted by Mentor to betheir most popular implant for over 2 decades. The obvious disadvantage is that if one particular area of concern needs special attention, a more specialized implant will give better improvement than this all-purpose breast implant.
Smooth Round Moderate Profile Style 1600 Sizing Chart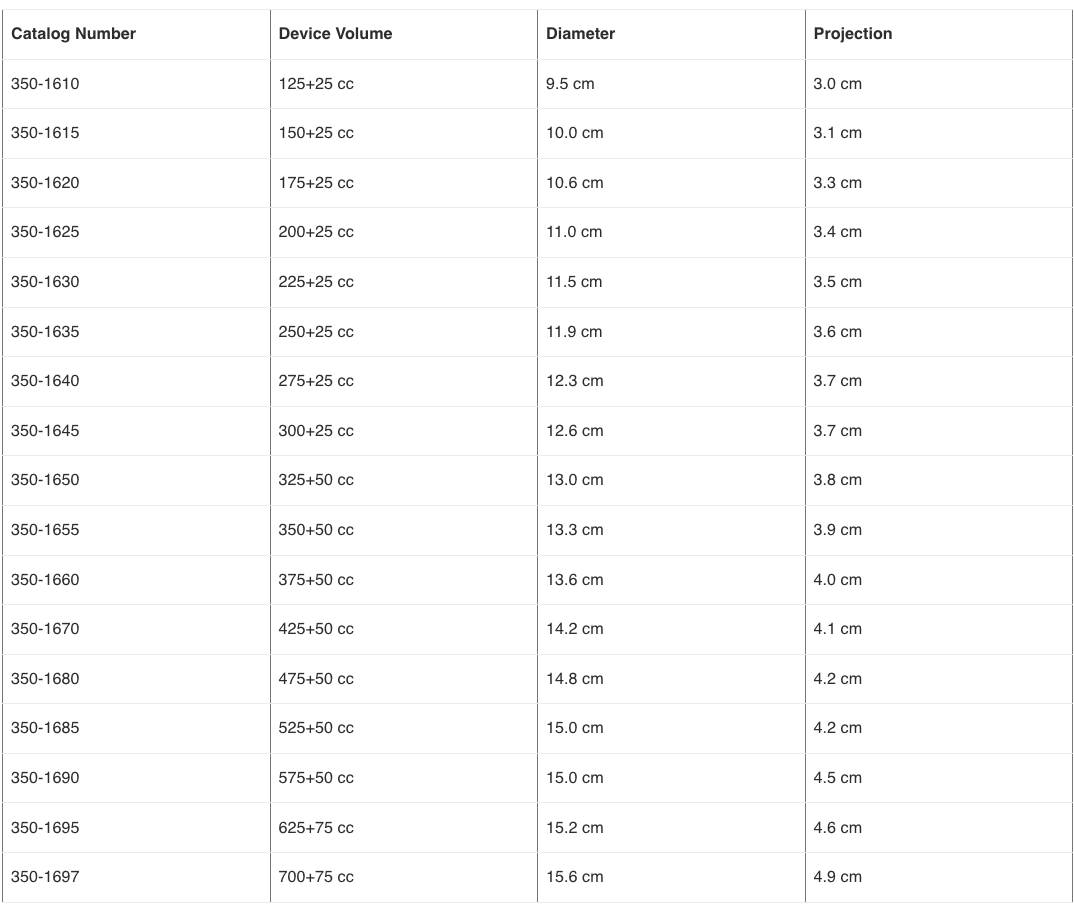
What are Round Saline Breast Implants? Particularly the Smooth Round Moderate Plus Profile Breast Implants, Style 2000 from Mentor? What are their advantages and disadvantages?
As the name suggests the Round Saline Breast Implants are round in shape (not anatomically shaped), have a moderate profile (not too low, and not too perky), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round moderate profile breast implants is a compromise between upper pole fullness, cleavage, and projection. It is a sort of “jack-of-all trades, master of none.” Because it does not emphasize one aspect of the implanted breast over another the augmentation takes place throughout, producing a full, rounded appearance. The Plus Profile Breast Implants do, however add a bit more projection, or the protuberance noted on side view at a slight expense to the width of the breast, and thus the cleavage. The obvious disadvantage is that if one particular area of concern needs special attention, a more specialized implant will give better improvement than this all-purpose breast implant.
Smooth Round Moderate Plus Profile Style 2000 Sizing Chart
Please Note: Individual implant dimensions may vary slightly in products of this type. Not all units will conform exactly to the dimensions noted above.
What was presented to the committee on safety of devices (MHRA)?
COMMITTEE ON SAFETY OF DEVICES – 20 NOVEMBER 2003 MEETING POLYURETHANE-COATED BREAST IMPLANTS
Background
Conventional breast implants consist of a silicone shell surrounding a silicone gel filling. The silicone shell can be smooth or textured. Silicone gel filled breast implants covered with a polyurethane foam coating were introduced to clinical use by an American manufacturer in the 1980s with the aim of reducing the risks of capsular contracture compared with those seen in conventional breast implants. They were withdrawn from the market in 1991 following concern that the coating might release a carcinogenic breakdown product. An equivalent product was subsequently reintroduced outside the USA and is currently authorised for marketing throughout Europe and is on sale in several European and South American countries. However, the product has not been available in the UK since 1993 and MDA issued advisory notices to draw attention to this in 1994 and 1996 (Annex 1).
In 2001, MDA responded to approaches from a European manufacturer and plastic surgeons on the anomalous position in the UK, compared with the rest of Europe, by preparing an updated analysis of the biological safety of the implants. This found that earlier conclusions on the carcinogenic risk still stood, but the benefits claimed of the polyurethane coating could not be substantiated by the evidence available. There was therefore no basis for altering the advice given in 1994 and 1996.
In July 2003, in response to the MDA report, the European manufacturer provided MHRA with a literature review, which concluded that the superiority of polyurethane-coated breast implants, in terms of capsular contracture rates, had been demonstrated.
Review of carcinogenicity
The carcinogenic risk arising from polyurethane-coated breast implants was assessed by the Committee on Carcinogenicity (COC) in 1991 and 1994. The COC concluded that the breakdown of the polyurethane coating over a number of years leads to the release of small amounts of the probable genotoxic carcinogen, 2,4-toluenediamine (2,4-TDA) into the tissues surrounding the implant. This gives rise to a small, unquantifiable carcinogenic risk.
No evidence has emerged since 1994 that would alter the COC’s conclusions, although MDA noted recent evidence for the in vivo genotoxicity of 2,4-TDA and for its presence in the tissue of implanted women.
The 2001 MDA Review of the biological safety of polyurethane-coated breast implants (Annex 2) summarised the relevant data, reported the opinions of the COC and presented a discussion of the various factors relevant to the carcinogenic risk assessment and their public policy implications.
Review of capsular contracture
The 2001 MDA review noted that there were some reports of a reduction in capsular contracture with polyurethane-coated breast implants but insufficient evidence was available to demonstrate conclusively their long-term benefits over other products. In addition, MDA noted that the necessity of using the particular polyesterurethane foam that gave rise to the release of 2,4-TDA had not been demonstrated. MDA had reviewed evidence published up to 2000, including that considered by Lübbers (2000) (Annex 3) and the case series by Vázquez (1999) which is appended to Annex 4.
The report provided by Polytech Silimed to MHRA in July 2003 (Annex 4) presented evidence for a lower, quantifiable rate of capsular contracture with polyurethane-coated breast implants. This report summarised data previously considered by MDA and reviewed two additional case series (Hester et al, 2001 (appended to Annex 4) and Brunnert, 2003 (Annex 5)).
Questions for the CSD
The CSD is invited to consider the following questions:
Are the benefits from polyurethane-coated breast implants substantial in terms of clinical outcome in respect of:capsular contraction rates;
aesthetic results?
Do the demonstrated benefits of polyurethane-coated breast implants outweigh the remote but unquantifiable carcinogenic risk, which has already been identified by the COC?
If polyurethane-coated breast implants were to be re-introduced to clinical use in the UK, what information on the risks and benefits should be available to:surgeons;
women considering implantation;
and what measures would be appropriate to provide this information?
Is the need for additional surgeries (reoperations) common after breast augmentation surgery with silicone breast implants?
Allergan’s Core Study showed a reoperation rate of 24% in primary augmentation and 35% in revision-augmentation in the first 4 years after receiving silicone implants. In primary augmentation cases, the three most common reasons for reoperations were capsular contracture, implant malposition, and ptosis (sagging). After revision-augmentation breast enlargement, the three most common reasons for additional surgery were capsular contracture, hematoma/seroma, and ptosis (sagging).Mentor’s Core Study showed a reoperation rate of 15% in primary augmentation and 3528% in revision-augmentation in the first 3 years after receiving silicone implants. For primary silicone breast augmentation patients, the three most common reasons for reoperation were severe capsular contracture, patient request for size/style change, andhematoma/seroma. In women who needed silicone revision-augmentation, the three most common reasons for additional surgery were severe capsular contracture, patient request for style/size change, and biopsy.
Is breast augmentation surgery the only surgery I will ever need to enhance my breasts?
The need for additional surgery after primary breast augmentation surgery is high, on order of 1 in 5 to 1 in 4 patients. Multiple factors account for this. Weighing down a breast with a silicone or saline breast implant may increase the chance for needing a breast lift down the road. Capsular contracture, or hardening of the breasts after breast enlargement surgery may require a revision depending on the severity of the problem. The rate of leakage and deflation also goes up with time, increasing the chances for implant removal. Patients may wish to address other cosmetic disturbances such as asymmetry, unfavorable scarring, shifting, etc. Allergan’s Core Study showed a reoperation rate of 24% in primary augmentation and 35% in revision-augmentation in the first 4 years after receiving silicone implants. In primary augmentation cases, the three most common reasons for reoperations were capsular contracture, implant malposition, and ptosis (sagging). After revision-augmentation breast enlargement, the three most common reasons for additional surgery were capsular contracture, hematoma/seroma, and ptosis (sagging).
Is the need for additional breast procedures common after breast enlargement surgery?
The need for additional surgery after primary breast augmentation surgery is high, on order of 1 in 5 to 1 in 4 patients. Multiple factors account for this. Weighing down a breast with a silicone or saline breast implant may increase the chance for needing a breast lift down the road. Capsular contracture, or hardening of the breasts after breast enlargement surgery may require a revision depending on the severity of the problem. The rate of leakage and deflation also goes up with time, increasing the chances for implant removal.
Is breast augmentation surgery painful?
The breast enlargement surgery itself should be painless, whether performed under sedation with local anesthetic, or general anesthesia. It is the postoperative period that some may find brings discomfort. Assuming the postoperative course is without complication, pain from breast augmentation surgery peaks the day after surgery, and diminishes over the course of the following three to four days to be tolerable enough without the use of narcotics. Of course, pain tolerance varies significantly from patient to patient. The 72-96 hrs time frame is a “ballpark” figure, and a reflection of personal experience. Several other important factors are crucial to consider in breast enlargement surgery. Pre-incisional administration of local or dilute local (called tumescent) anesthetic greatly diminishes postoperative breast enlargement surgery pain. Postoperative pain and tenderness can be further affected by breast implant placement position. Sub-muscular placement or “dual plane” placement may cause substantially more discomfort than sub-glandular placement because of muscle dissection. If a skin excision is necessary, pain may be more pronounced. The injection of local anesthetic at the conclusion of breast augmentation surgery greatly diminishes postoperative discomfort. In addition, a more effective multiple intercostal nerve block may be performed. Finally, a small catheter may be placed, within the breast implant pocket, for the purpose of delivering local anesthetic in the post breast enlargement surgery period. This has been shown in studies to help significantly with postoperative discomfort. In short, breast enlargement surgery is very well tolerated in most patients.
What type of incision should I chose when undergoing breast enlargement surgery? What are the advantages and disadvantages of each?
The inframammary approach to breast augmentation surgery allows your plastic surgeon the greatest visibility while dissecting an implant pocket, and as such, allows greater precision and control of symmetry. The inframammary incision is placed in the fold under the breast, in the breast fold, and tends to be more noticeable for two reasons. It is typically larger than other types of breast incisions. It has a tendency to migrate as the implant settles up or down, and its final resting place is less predictable.The periareolar incision goes around the nipple. It is less noticeable, smaller than the inframammary incision, and if a lift is required later in time, it may be utilized again. Its disadvantages in breast enlargement surgery are a higher infection rate (because the cut goes through breast tissue), possible difficulty in breastfeeding, and the risk of decreased sensation in breast skin and nipple sensation.Placing the incision in the axilla (underarm) allows for a smaller incision. It is useful when there is not much breast tissue present to begin with, and thus no prominent breast fold, and when the nipple-areola complex is small. It is not as well concealed as the periareolar incision, gives less control in terms of the ability to feel around the breast pockets in assuring symmetry, and placement of silicone breast implants will necessitate a larger incision which may be less pleasing.The peri-umbilical incision is placed at the top of the belly-button. Its obvious advantage is a lack of scars on, around, or near the breasts. Its disadvantages are blind dissection, making asymmetry more common, and making the likelihood of one side being submuscular and the other subglandular more likely. If an undesirable result is obtained, a new incision will be needed to correct the problem. It may also damage the breast implants, and cannot be used with pre-filled silicone breast implants.
Is infection common after breast enlargement surgery, what are the consequences, and what is the treatment?
Any surgery, in any discipline carries a risk of infection. The risk is calculated based on the degree of contamination for a particular operation. Breast augmentation is considered a “clean” surgery, and carries an overall infection rate of less than two percent. If infection should take place, it will most often affect one or more of three patterns, assuming there is no disseminated spread, and the infection remains localized. Infection can occur in the skin, in the soft tissue surrounding the implant, and in the form of a pus pocket. Skin infection will usually respond to oral antibiotics. Soft tissue infections surrounding the breast implant may respond to oral antibiotics, will sometimes require intravenous antibiotics, and in other cases need to be treated with implant removal. If the infection should progress to, or start out as an abscess (pus-pocket), the only treatment that will be effective in treating the infection and preventing more serious systemic complications is drainage of pus and breast implant removal. Toxic Shock Syndrome (TSS) may result from the presence of a foreign body (breast implant in this case) in the setting of an infection, and is a truly life-threatening condition that needs to be addressed immediately. It is marked by high fever, nausea and vomiting, diarrhea, light-headedness and possibly loss of consciousness, and a diffuse rash. The treatment is timely institution of IV antibiotics, and breast implant removal. If the breast implant is removed, the infection should be treated, the inflammation allowed to resolve, and a new implant placed weeks down the road.
What is an inframammary incision with respect to breast augmentation?
The inframammary approach to breast augmentation surgery allows your plastic surgeon the greatest visibility while dissecting an implant pocket, and as such, allows greater precision and control of symmetry. The inframammary incision is placed in the fold under the breast, in the breast fold, and tends to be more noticeable for two reasons. It is typically larger than other types of breast incisions. It has a tendency to migrate as the implant settles up or down, and its final resting place is less predictable.
Are silicone breast implants safe?
The controversy over the safety of silicone breast implants was founded more on the desire to wage successful litigation with lucrative awards, than on science. It was raised more to shift public opinion in the direction of paranoia and “uncover” some sinister plot while building a “solid” career in journalism, than to extricate fact from events. The battle was a classic of example of lies repeated long enough, loud enough, and by enough visible and “educated” people to sway the opinion of juries from fact to confabulation. One thing for certain, science, fact, or truth had nothing to do with the conduct of the players. Money, greed, and ambition had everything to do with it.Let’s begin by noting first that nothing in life is guaranteed. In allowing oneself to be implanted with breast prosthesis, a patient is permitting an unnatural act upon herself. A foreign substance is placed into a consenting body. Any foreign substance, or object will be regarded by the organism as just that, a foreign body. Any such foreign substance will incite a response, be it a breast capsule, or a more violent immune response. The question should then be restated, “how unsafe is a silicone implant?” The parties involved in condemning the use of silicone without a shred of scientific evidence effectively cheated millions of women afflicted with breast cancer out a perfectly good breast reconstruction, unless they were willing to enroll in a study. To add to the charade, millions more who had been implanted, but symptom free, had their breast implants removed. To answer the question of breast implant safety, we need to look back to the beginning…In the 1940’s, convinced of the American GI’s penchant for large breasts, Japanese prostitutes would inject their mammary glands with various materials of dubious origin. The substances included sponge, paraffin, and silicone. Fast forward to the 1960’s. Doctors Gerow and Cronin began implanting silicone breast prostheses in Houston. In 1976, the silicone breast implant, along with all other medical devices came under the jurisdiction of the Food and Drug Administration in an amendment of the Federal Food, Drug, and Cosmetic Act. Because silicone implants were in use for years prior to the enactment of the amendment, the only requirement imposed on their producers was the provision of safety data on FDA’s demand. In 1977, the first settlement from Dow was furnished to a patient with “pain and suffering” as a result of implant rupture requiring multiple revisions. In the 1980’s, Ralph Nader’s Public Citizen Health Research Group began implicating silicone breast implants in the etiology of breast cancer. As is the case with many of Mr. Nader’s allegations today, the statements were based on anecdotal “evidence.” This is the type of proof the scientific community regards as equivalent to no proof, and the type our attorney friends call hearsay. As a result of the dissemination of this propaganda, in 1982, the Food and Drug Administration suggested that silicone breast implant producers demonstrate conclusively the safety of silicone breast implantation, placing the device into category III. In 1984, Maria Stern was awarded 211k in compensatory, and 1.5m in “punitive” “damages.” The charade put on by Dan Bolton, her “attorney,” included the testimony of theorizing “experts,” who through their “expertise,” but without the burden of true scientific research or proof of any kind, linked breast cancer with silicone. The 1982 category III re-classification proposal materialized in 1988, with the premarket approval applications (PMA) due date set in July of 1991. The FDA would then review the applicants’ data over the following six months. Prior to any such due process, however, the case for the silicone implant was “reviewed”, no doubt after exhaustive “research,” by Connie Chung on “Face to Face.” Sensationalism sells, and Connie’s brand sold particularly well. Without regard for truth, and based entirely on a handful of anecdotal reports and cases, she effectively paraded women who claimed to contract autoimmune disease from breast implants, in the process fueling the baseless paranoia. It is quite unfortunate, and very sad that these patients were sick. It is even more unfortunate that a third party was able to use them to capitalize on emotions stirred up in the public. The “good” journalist never stopped to think that of the millions of women who had silicone implants placed, some were bound to be affected by autoimmune disease at the rate of the general population. Truth, however, seldom makes careers. At around the same time Ralph Nader’s “Public Citizen” jumped on the band wagon, aided Dr. Sidney Wolfe, stoking the flames and pressing in courts, while Representative Ted Weiss headed a Congressional Hearing on the safety of silicone breast implants. It is interesting that a court order from the Stern hearing barred the presentation of certain evidence for publi
Can any patient receive the latest generation cohesive or gummy-bear silicone implants?
Patients who seek breast reconstruction, revision or secondary breast augmentation, or a first time breast enlargement may enroll in the trial. A list of prohibited medical conditions for participating in the study must be satisfied, and patients must agree to a ten-year follow-up.
Is loss of sensation from the skin of the lower pole of the breast common after breast enlargement surgery, what are the consequences, and what is the treatment?
Nerves that supply sensation to the nipple and surrounding (areolar) skin may be cut or stretched during surgery. Most often this is not the case, and if sensation is lost, it generally due to stretching of the nerves, and will eventually recover. Permanent loss of nipple sensation, however, is always a possibility. Sometimes the nipple skin will become more sensitive to any form of stimulation, which may be due to nerve involvement, or to the stretching of the skin by the breast implant. This, too, is normally temporary. Usually, it is not the nipple that loses sensation, but the nerves to the skin of the lower part of the breast, especially on its outside edge. This is because the nerves giving sensation to that part are most frequently encountered, and manipulated in some way. As with nipple sensation, this usually returns, but not always.
Is loss of nipple sensation possible after breast augmentation surgery?
Nerves that supply sensation to the nipple and surrounding (areolar) skin may be cut or stretched during surgery. Most often this is not the case, and if sensation is lost, it generally due o stretching of the nerves, and will eventually recover. Permanent loss of nipple sensation, however, is always a possibility. Sometimes the nipple skin will become more sensitive to any form of stimulation, which may be due to nerve involvement, or to the stretching of the skin by the breast implant. This, too, is normally temporary. Usually, it is not the nipple that loses sensation, but the nerves to the skin of the lower part of the breast, especially on its outside edge. This is because the nerves giving sensation to that part are most frequently encountered, and manipulated in some way. As with nipple sensation, this usually returns, but not always.
Is it better to use a low profile breast implant or a high profile breast prosthesis in breast enlargement surgery?
There are several advantages to the use of high profile breast implants. First, just as the name suggests, for any given fill volume, high profile breast implants will implant more projection or profile when compared to moderate or “normal” breast prosthesis, and most certainly more than low profile, or anatomically shaped breast implants. The way to picture this is that if you had a cone with a highly sitting tip versus a cone with a wide base, the narrower, taller cone would point more (think of Madonna’s show bra!) and thus give more projection to the breast. What this means is that women with a narrower, smaller chest wall can still have larger breasts. The advantage to some women, and disadvantage to others comes from a basic difference in perception as what a natural breast should look like. If a patient prefers a highly “perky,” high profile, or well projecting breast, the high profile breast implant would be considered ideal. High profile breast implants would not be ideal to place in a patient who prefers natural, gently sloping, and slightly ptotic (hanging) breasts, or in a client who is large and wide chested. Placing full profile breast implants in the case of a wide chest would impart a very unnatural “double cone” appearance. For women with a mid-range chest-wall diameter the choice is one of partiality. That is, the decision has to be made between projection, and cleavage. This is because lower profile implants with a wide base will naturally fill up the inner, otherwise known as the medial breast, and produce cleavage. Finally, it is mostly the anecdotal opinion of some authorities that full or high profile implants tend to generate less rippling.
Does breast enlargement surgery, or do breast implants interfere with mammography?
Breast implants, whether silicone or saline interfere with mammography. Special views need to be obtained by radiologists experienced in the Eklund technique to visualize any abnormalities, however breast implants also project breast tissue from behind its base making any masses easier to feel. If you need to obtain a mammogram after your breast enlargement surgery, your radiology-mammography technologist must be informed about your breast implants so that in addition to obtaining additional views, special techniques may be used to minimize the chances for implant rupture.
Should I have a mammogram prior to have breast augmentation surgery?
There are guidelines for routine screening mammography established by the National Cancer Institute. The factors that are very important in determining the appropriate age for a first, and subsequent mammograms are age, patient history, and family history. Even if the guidelines suggest that you are too young, say 10 years younger than recommended, for mammography, it may be a good idea to have a “baseline” mammogram before, and six months after breast enhancement surgery. This is because breast enlargement surgery makes scars on the inside of the augmented breast which can calcify and fool mammographic examiners as to the cause of the calcium deposits. This would then lead to an unnecessary biopsy, which has the potential to add scars, distort breast tissue, or cause implant rupture. For NCI information on breast cancer screening, please see below.
Breast Cancer Screening
Different tests are used to screen for cancer.
Three tests are commonly used to screen for breast cancer:Mammogram
Clinical breast exam (CBE)
Breast self-exam (BSE)
New screening tests are being studied in clinical trials.MRI (magnetic resonance imaging)
Tissue sampling
Some screening tests are used because they have been shown to be helpful both in finding cancers early and in decreasing the chance of dying from these cancers. Other tests are used because they have been shown to find cancer in some people; however, it has not been proven in clinical trials that use of these tests will decrease the risk of dying from cancer.
Scientists study screening tests to find those with the fewest risks and most benefits. Cancer screening trials also are meant to show whether early detection (finding cancer before it causes symptoms) decreases a person’s chance of dying from the disease. For some types of cancer, finding and treating the disease at an early stage may result in a better chance of recovery.
Clinical trials that study cancer screening methods are taking place in many parts of the country. Information about ongoing clinical trials is available from the NCI Web site.
Three tests are commonly used to screen for breast cancer:
1.) Mammogram
A mammogram is an x-ray of the breast. This test may find tumors that are too small to feel. A mammogram may also find ductal carcinoma in situ, abnormal cells in the lining of a breast duct, which may become invasive cancer in some women. The ability of a mammogram to find breast cancer may depend on the size of the tumor, the density of the breast tissue, and the skill of the radiologist.
2.) Clinical breast exam (CBE)
A clinical breast exam is an exam of the breast by a doctor or other health professional. The doctor will carefully feel the breasts and under the arms for lumps or anything else that seems unusual.
3.) Breast self-exam (BSE)
Breast self-exam is an exam to check your own breasts for lumps or anything else that seems unusual.
If a lump or other abnormality is found using one of these 3 tests, ultrasound may be used to learn more. It is not used by itself as a screening test for breast cancer. Ultrasound is a procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram.
New screening tests are being studied in clinical trials.
MRI (magnetic resonance imaging)
MRI is a procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI). Screening trials of MRI in women with a high genetic risk of breast cancer have shown that MRI is more sensitive than mammography for finding breast tumors.
MRI scans are used to make decisions about breast masses that have been found by a clinical breast exam or a breast self-exam. MRIs also help show the difference between cancer and scar tissue. MRI does not use any x-rays.
Tissue sampling
Breast tissue sampling is taking cells from breast tissue to examine under a microscope. Abnormal cells in breast fluid have been linked to an increased risk of breast cancer in some studies. Scientists are studying whether breast tissue sampling can be used to find breast cancer at an early stage or predict the risk of developing breast cancer. Three methods of tissue sampling are under study:
Fine-needle aspiration: A thin needle is inserted into the breast tissue around the areola (darkened area around the nipple) to withdraw cells and fluid.
Nipple aspiration: The use of gentle suction to collect fluid through the nipple. This is done with a device similar to the breast pumps used by nursing women.
Ductal lavage: A hair-size catheter (tube) is inserted into the nipple and a small amount of salt water is released into the duct. The water picks up breast cells and is removed.
Screening clinical trials are taking place in many parts of the country. Information about ongoing clinical trials is available from the NCI Web site.”
Should I massage my breasts after breast enlargement surgery?
There have not been studies demonstrating conclusively, any benefit to massaging your breasts, however many surgeons feel that it helps diminish the chance for capsular contracture. Your body’s natural response to any implant is scar formation, the scar envelops the implant and may contract with time, causing firm capsule formation, and possibly palpable or even visible flaws. Massage is performed in the hopes of enlarging the space in which the implant moves about, thereby enlarging the size of the capsule. It should be performed slowly, and deliberately. It should be performed symmetrically, that is displacing the implant equally on both sides with each movement/repetition. Slowly press the implants inward, and hold for 10 seconds, release and repeat 10 times. Repeat the same pressing downward, and upward for the same number of repetitions.
What is the McGhan style 410 breast implant, what are its features, and when should it be used?
The Style 410 McGhan Breast Implant is composed of a gel that is considered to be more cohesive than prior silicone breast implant designs. It is engineered to allow simulate the appearance, and tactile sensation of a natural breast. Its BIOCELL® textured surface is said to facilitate a gentle degree of tissue adherence. As explained before, this may keep the implant within the dissected surgical breast pocket, whether submuscular, or subglandular. The texture of the Style 410 breast implants may also serve to diminish the degree of capsular contracture. The fill used to construct the Style 410 implant consists of a more cohesive silicone, which not only more closely approximates the look and feel of natural breast tissue, but is less prone to significant soft tissue spread should a leak occur. The patented barrier within the implant is stated to minimize silicone diffusion out of the implant shell. This implant is also available in a wide variety of sizes, and thus dimensions, for attaining the desired result with respect to cleavage (medial fullness), upper breast pole fullness, and breast protrusion (projection).
What various sizes and shapes do the 410 “gummy bear” silicone gel implants come in?



What are MemoryGel™ Smooth Round High Profile Breast Implants? What are their advantages and disadvantages?
As the name suggests the MemoryGel™ Smooth Round High Profile Breast Implants are round in shape (not anatomically shaped), have a high profile, giving them more of a bulge on side view (the most perkiness for your buck), and are encased in a smooth silicone elastomer shell. The advantages of using textured versus smooth breast implants are discussed in another section of this text, but are more related to physician preference, and beliefs regarding skin palpability than scientific data. The biggest advantage of the round high profile implants is this protuberance, considered youthful by many patients, and plastic surgeons. Another considerable advantage is the ability to use higher volume implants in patients with narrow chest walls because the width of the implant base is narrower. In addition, the use of silicone allows for the use of the MemoryGel™ Smooth Round High Profile Breast Implants in breasts with less skin and soft tissue cover. Some physicians prefer to use the larger size breast implants in moderate breast ptosis (sagging breasts) to avoid extra incisions. Use of silicone implants, because of their consistency, is ideal in such cases, especially when the lower pole of the breast has little soft tissue cover. The particular proprietary polymer employed in the Mentor devices is a cohesive gel that is purported to have more consistency such that even in cases of ruptured breast implants it is said not to leak as fast or as much. The disadvantages include a sacrifice of cleavage, owing to the decrease in the base diameter of the implant, and a drop in upper pole fullness for the same reason. In addition some women find the look imparted by the high profile breast implant too perky.
What is the thinking behind making the cohesive or gummy bear implant anatomically contoured?
There is a gentle curve to the breast. The top portion protrudes less, the bottom more, creating a gentle slope. The argument for a shaped gummy bear breast implants makes sense intuitively only. That is, a formed breast implant will maintain its shape at the top and not sag to the bottom, as would a more liquid implant. This is cannot be true, not because I disagree with it but because liquids are non-compressible, as are solids. A more liquid-like implant may be more prone to deformation through gravity, but all that would cause is rippling, as the down pulling silicone would exert traction on the top portion of the implant.
Is it possible for “Gummy Bear Breast Implants” to rupture?
Although probably less likely than other type of breast implant rupture, the “gummy bear silicone breast implant” can rupture or tear. After the implantation of thousands of such breast implants in Europe, the reported incidence of rupture is confined to several cases. This may be because most cases of rupture may not be picked up owing to the “gummy bear” consistency of the silicone gel. The consequences of leaking “gummy bear breast implants” are not well known, and there are studies going on right now to determine the safety of this cohesive silicone gel implant. Intuitively, the silicone “gummy bear” implant should not migrate as much as would be expected of the more liquid older generation silicone breast implants.
What are Cohesive Gel Breast Implants (“Gummy Bear Breast Implants”)?
Cohesive gel breast implants were formulated with a thicker form of silicone (viscous silicone polymer as a result of more extensive cross-linking) resulting in three ideal properties; less silicone “run-off,” in the case of rupture, no rippling, and maintenance of more precies form. If you slice into a third generation cohesive gel implant from Mentor and turn the two halves over, the silicone will not leak, drip, or run. You can see how this would be advantageous in case of rupture. It should be mentioned that “cohesiveness” is an attribute that is present in most of the older generation implants, so technically using such implants may be described as using cohesive gel implants. Recently, the latest generation of silicone breast implants was dubbed cohesive in the media. This is true, but misleading, once again, because all silicone implants are cohesive to a certain extent. What most consumers are interested in when they refer to cohesive implants are the so called “gummy bear implants,” or the latest generation of highly cross-linked silicone breast implants. So, back to the two most favorable properties of the so called “gummy bear breast implants.” They are more solid, and they do not ripple. This makes them ideal for thin skinned patients, because soft tissue cover (breast tissue or fat) is not as important in concealing the implant since the formed or cohesive gummy bear breast implant should hold its shape, and not ripple. It is also ideal in breast reconstruction for the same reason, and because large incisions are already present from the mastectomy (breast removal).
Why is the cohesive “gummy bear” implant being investigated?
FDA regulations mandate that all new devices be subjected to rigorous clinical trials to determine their safety regardless of how safe they may be thought, or how many regulatory bodies have approved them outside of the United States.
How can I tell if my cohesive gel (“gummy bear”) breast implants have ruptured, or are leaking?
Unfortunately, this is a question that can be answered only in speculation, without hard medical evidence. Presumably the more solid, formed nature of silicone “gummy bear” implant would not allow as much leakage, or as rapid a spill as a conventional silicone implant. This means that a patient would not be able to pick this up on routine self-inspection very easily. Having stated this a “gummy bear” implant patient who develops soreness and possibly enlarged lymph nodes in the armpit should promptly report to her surgeon.
Unfortunately, this is a question that can be answered only in speculation, without hard medical evidence. Presumably the more solid, formed nature of silicone “gummy bear” implant would not allow as
The breast enlargement surgery itself should be painless, whether performed under sedation with local anesthetic, or general anesthesia. It is the postoperative period that some may find brings discomfort. Assuming the postoperative course is without complication, pain from breast augmentation surgery peaks the day after surgery, and diminishes over the course of the following three to four days to be tolerable enough without the use of narcotics. Of course, pain tolerance varies significantly from patient to patient. The 72-96 hrs time frame is a “ballpark” figure, and a reflection of personal experience. Several other important factors are crucial to consider in breast enlargement surgery. Pre-incisional administration of local or dilute local (called tumescent) anesthetic greatly diminishes postoperative breast enlargement surgery pain. Postoperative pain and tenderness can be further affected by breast implant placement position. Sub-muscular placement or “dual plane” placement may cause substantially more discomfort than sub-glandular placement because of muscle dissection. If a skin excision is necessary, pain may be more pronounced. The injection of local anesthetic at the conclusion of breast augmentation surgery greatly diminishes postoperative discomfort. In addition, a more effective multiple intercostal nerve block may be performed. Finally, a small catheter may be placed, within the breast implant pocket, for the purpose of delivering local anesthetic in the post breast enlargement surgery period. This has been shown in studies to help significantly with postoperative discomfort. In short, breast enlargement surgery is very well tolerated in most patients.
What are Hydrogel Breast Implants (Hyaluronic-Acid Filled Breast Implants)?
This type of breast implant is not available in the U.S., and has been taken off the UK market. This is not because it is unsafe, but because not enough data exists to prove that it is safe. The same substance that is used to manufacture Juvaderm®, Restylane®, Captique®, Hylaform®, etc., was used to manufacture the Hydrogel-filled implant. That is, it was used as the filler in the standard silicone elastomer shell. Since the implants are not available, further discussion is unwarranted. For further information, please read the information put forth by the British MHRA (Equivalent of FAD, except for the food part).“Hydrogel filled breast implants are no longer available in the UK.Hydrogel breast implants consisted of a silicone elastomer shell containing hydrogel filler. Hydrogels are polymeric materials that have the ability to swell in water without dissolving and retain water within their structures.Until December 2000, there were two models of hydrogel filled breast implants on the UK market.Because of continuing concern over the safety of breast implants, the MDA (Medical Devices Agency, a forerunner to the MHRA) carried out a series of investigations into the safety of the various filler materials used in the implants available in the UK. The MDA’s investigations into hydrogel-filled breast implants revealed inadequacies in the manufacturers’ biological safety assessments and concluded that there was not enough information to fully assess the safety of either of the hydrogel filler materials. When they were made aware of the results of the MDA’s reviews, both manufacturers decided to withdraw their hydrogel-filled breast implants from the UK market in December 2000 as a precautionary measure, pending further studies to establish the safety of the filler materials.It must be emphasised that no definite risk has been identified. The concern lay only with the way the safety of the hydrogel fillers had been assessed. The MHRA is continuing to monitor the safety of these implants and, based on the currently available evidence, does not recommend that women with hydrogel-filled breast implants have them removed unless they are experiencing problems.Women who think that they may have hydrogel breast implants and are concerned, should contact the surgeon who carried out their operation, their GP or get advice from NHS Direct on 0845 4647 or in Scotland the Scottish NHS Helpline on 0800 224488.Clinicians and members of the public should continue to report adverse incidents to the MHRA.
Information specific to each hydrogel filled breast implant:
PIP Hydrogel breast implants were made in France and supplied in the UK by Clover Leaf Products Ltd. Approximately 4000 women in the UK received these implants since they were first introduced in 1994. The removal of these implants from the UK market was the subject of Device Alert DA 2000(07). This noted concerns about uncertainty over the metabolic fate of the filler material and about pathological changes seen in rats following implantation of the filler material that suggested the possibility of a systematic effect. The CE-marking was removed in 2002. In May 2005 the Committee on Toxicity (COT)1 reviewed two animal studies commissioned by PIP. Although some effects were seen in the kidneys of the tested animals, in a statement (external link) prepared in January 2006, the Committee concluded that these results suggested that exposure to the hydrogel filler will not lead to toxic effects in women with these implants. There is therefore no indication for a proactive explantation programme. The Committee did, however, express concern about the lack of long-term follow up of women with breast implants.The MHRA has received adverse incident reports of breast swelling associated with the presence of fluid in the implant pocket around PIP Hydrogel breast implants. The MHRA continues to monitor the safety of PIP Hydrogel implants and, based on the currently available evidence, does not recommend that women with these hydrogel-filled breast implants should have them removed unless they are experiencing problems. However clinicians who remove implants due to swelling (with or without suspected implant rupture) should consider having the implants weighed and photographed, carrying out histology of the surrounding capsule and cytology of any collected fluid; such cases should be reported to the MHRA.NovaGold™ breast implants were made in Germany and supplied in the UK by Somatech Medical Ltd. Approximately 250 women in the UK received these implants since they were first introduced in 1996. It is estimated that around 80% of these implants were implanted by the private sector for cosmetic reasons. The removal of these implants from the UK market was the subject of Device Alert DA 2
Besides silicone breast implants, or saline breast prostheses, what else is out there?
Cohesive Gel Breast Implants (Gummy Bear Breast Implants), Hydrogel Breast Implants (Hyaluronic-Acid Filled Breast Implants), PIP Hydrogel Breast Implants, PIP Saline Breast Implants, Trilucent Breast Implants (Peanut Oil Breast Implants, Soy Oil Breast Implants, Soya Oil Breast Implants, Soybean Oil Breast Implants), Hydrophilic Polyacrylamide Gel Breast Implants.
What is implant exposure or breast implant extrusion after breast enlargement surgery, what are the consequences, and what is the treatment?
The exact risk percentage may be found for both the Allergan, and the Mentor studies in related questions. The problem most often is the result of inadequate soft tissue (breast and skin) overlying the breast implants. It is unusual in cases of primary augmentation, and is more commonly seen in the compromised soft tissue of the reconstructed breast.If the extrusion if only threatened, and no implant is actually showing at the time of detection, a salvage procedure may be done to improve the soft tissue cover atop the implant. If the implant is exposed, many plastic surgeons will not attempt salvage, as by definition, and exposed implant is a contaminated implant. The breast implant will be removed, patient allowed to heal, and implant replaced at a later time.
How can the palpability of a breast implant be reduced in breast enlargement surgery?
A breast implant is more likely to be noted on manipulation of the breast when they are too big for the breast and soft tissue present, when they are over, rather than under the muscle, and when they are textured. Ensuring a small enough base width, good soft tissue cover with a submuscular or dual-plane placement, and using smooth breast implants will decrease the risk for this.
What is palpable or visible breast implant rippling after breast augmentation surgery, what are the consequences, and what is the treatment?
Breast implants have fold flaws. Because of the consistency of silicone gel, and predetermined, and optimal filling, the flaws may be less pronounced in such breast implants. Saline breast implants may produce scalloping and folding that is made more pronounced by overfilling, or under-filling breast implants. The visibility or palpability of breast implant rippling is accentuated by lack of breast tissue, fat tissue, or muscle tissue between the breast implants, and their overlying skin. The solutions to the problem include replacement with the latest generation cohesive gel implants or augmentation of the soft tissue covering the breast implants with flaps.
What are some of the unestablished consequences of implant rupture?
Problems not specific to silicone breast implant rupture include breast hardness, a change in breast shape or size, and breast pain. Gel migration has also been infrequently reported to areas far removed from the breast like the underarm, abdominal wall, arm, groin, and chest wall. Inflammation around nerves, granuloma and scar formation have been associated with this. Silicone migration has also been rarely reported to lymph nodes, in absence of rupture.
Is breast implant rupture common after breast augmentation surgery, what are the consequences, and what is the treatment?
Breast enlargement implants collapse when either saline or silicone that is contained within them leaks out through a broken valve (saline breast implants only), or a tear in the implant shell (both silicone and saline implants). Saline is reabsorbed rapidly, and a dramatic change in size will be noted by the patient within days. Silicone breast implants rupture may go undetected, may present with a slight change in shape from one breast to the other, or may have a more pronounced difference in size between the two breasts. Many rupture events go unexplained, but may be due to inherent defects in the implant with worsening over time. Known reasons for deflation consist of overfilling or underfilling saline breast implants, obvious causes such as undetected damage to the breast implants in surgery (tears, needle holes, etc.), trauma whether via accident, aggressive manipulation during intimate contact, or during mammography, the scar around the breast implant squeezing to the point of rupture (capsular contracture), attempted rupture of such contracture via closed capsulotomy. Consequences of saline rupture are cosmetic only. Many ill-effects have been attributed to silicone leak, however, in spite of opportunistic attempts at destroying scientific method of research as it applies to silicone breast implants, local inflammation and difficulty in extracting all of the leaked silicone gel has been the only real issue. Obviously, the treatment in such cases is implant removal and replacement.
What type of implants should I ask my surgeon to use in enlarging my mammaries through breast augmentation surgery?
There are multiple different types of implants available for use in breast enlargement. In addition multiple permutations of the various prosthesis types yield a fairly wide array of choices in terms of material, shape, coating, size. In addition, each of the two currently approved breast prosthesis manufacturers in the United States (Inamed™ and Mentor™) makes their own version of each implant with the varying characteristics. The availabilities are as follows. Each implant is encased in a silicone elastomer shell. The shell may be smooth, or textured. The shell may be shaped like a tear-drop, also referred to as anatomical, or the shell may be perfectly round. Each configuration of the round implant may have a different degree of projection even if the amount of volume contained within is the same. As an example, one style of implant may have a volume of 500cc and a projection of 3.6cm, while another, may have a volume of 500cc but a projection of 4.7cm as a result of a narrower base. The filling material of a breast implant may be silicone, or saline, or both in the case of some implants used for breast reconstruction. The size of an implant may vary to over 1000cc, though volumes of that size are not currently approved in the U.S. and must be procured through companies in Europe or South America.
Do breast implants come with a warranty, if so what does the warranty cover, and what is excluded?
There are two FDA approved manufacturers of breast implants within the United States, Mentor™ and Allergan™. Each corporation has its own breast prosthesis warranty, with options for more extensive coverage. Both policies are quoted below.“Mentor’s free Lifetime Product Replacement Policy involves the free lifetime product replacement for its gel-filled and saline-filled breast implants, worldwide. When implant replacement is required and the Mentor Product Replacement Policy applies (see below), Mentor will provide, throughout a patient’s lifetime, the same or similar Mentor breast implant at no cost. If a more expensive product is requested, Mentor will invoice the surgeon for the price difference.The Mentor Standard Advantage Limited Warranty is free of charge to all patients who are implanted with Mentor gel-filled or saline-filled breast implants in the United States and Puerto Rico. When the limited warranty applies, Mentor provides the following:Financial assistance: For the first ten years following a breast implant procedure, Mentor will provide financial assistance up to $1200 to help cover operating room, anesthesia, and surgical charges not covered by insurance. Financial assistance applies to covered events only (see below). Operating room and anesthesia charges will be given payment priority. In order to qualify for financial assistance, the patient will need to sign a Release Form.Free contralateral (opposite side) implant replacement upon surgeon request. Non-cancelable terms.The Mentor Enhanced Advantage Limited Warranty is an optional limited warranty available for women who are implanted with Mentor gel-filled or saline-filled breast implants in the United States and Puerto Rico. To be eligible, the Mentor Enhanced Advantage Limited Warranty must be purchased for an enrollment fee of $100 within 45 days from implantation. When the warranty applies, Mentor provides the following: Financial assistance: For the first ten years following a breast implant procedure, Mentor will provide financial assistance up to $2400 to help cover operating room, anesthesia, and surgical charges not covered by insurance. Financial assistance applies to covered events only (see below). Operating room and anesthesia charges will be given payment priority. In order to qualify for financial assistance, the patient will need to sign a Release Form. Free contralateral implant replacement upon surgeon request.Non-cancelable terms.With both the Mentor Standard Advantage and Mentor Enhanced Advantage Limited Warranties, it is important for the patient to also maintain her own records to ensure validation of her enrollment.
Products Covered
The Mentor Standard Advantage Limited Warranty coverage applies to all Mentor gel-illed and saline-filled breast implants that are implanted in the United States and Puerto Rico, provided they have been:Implanted in accordance with the Mentor package insert, current to the date of implantation, and other notifications or instructions published by Mentor; andUsed by appropriately qualified, licensed surgeons, in accordance with accepted surgical procedures.
Events Covered
The Mentor Lifetime Product Replacement Policy, and the Standard Mentor Advantage and Enhanced Advantage Limited Warranties coverages apply to the following:Rupture due to localized stress, folding, manufacturing defect, patient trauma, or unknown causeOther loss-of-shell integrity events, such as surgical damage may also be covered by these programs. Mentor reserves the right to determine if specific, additional events should be covered.
Events Not Covered
The Mentor Lifetime Product Replacement Policy and the Mentor Standard Advantage and Enhanced Advantage Limited Warranties coverages do not apply to the following: Removal of intact implants due to capsular contracture, or wrinkling. Loss of implant shell integrity resulting from preoperative procedures, open capsulotomy, or closed compression capsulotomy procedures. Removal of intact implants for size alteration.
Filing for Financial Assistance
To file a Mentor Advantage claim for product replacement and/or financial assistance, the surgeon must contact the Mentor Product Evaluation Department at 1-866-250-5115 prompt #1 prior to replacement surgery.For financial assistance claims, a patient-specific Release form will be generated that the patient must sign and return.For either replacement or financial assistance claims, the surgeon must send the explanted, decontaminated Mentor breast impl
What type of surgical techniques are not appropriate for plastic surgeons to use in breast augmentation surgery for risk of implant shell rupture?
The filling of saline breast implants with anything other than saline, bathing saline breast implants in iodine solution, penetration of the saline breast implant shell during injection, utilizing more than one saline breast implant per side, attempts at reshaping the saline breast implant can all compromise the integrity of the saline breast implants’ shells and lead to rupture or leak.
How should I care for my incisions after breast enlargement surgery?
Dr. Gerzenshtein’s recommendations to his patients are found below. Consult with your own surgeon for his ore her specific instructions. After surgery you will be placed in a bra, possibly with an ace wrap above it. If the ace is present, it was applied to control pocket position in cases where implant position was made submuscular and the muscle was not entirely divided at its lower edge, this means that the ace wrap must be re-applied exactly the way you were instructed. The ace wrap along with the dressing, will removed for the first time 24 hours after surgery, in the office. After the initial dressing change, dressings should be changed twice a day in the following manner: Remove old dressing and wash or shower, after drying, apply new dressing; ABDs followed by bra, then ace if indicated. When the edges of the steri-strips become frayed trim them. With time, as very little is left behind, they may be removed (usually 2-4 weeks). It is not routine to have drains placed at the time of surgery, however, at times, if bleeding is diffuse, and cannot be addressed via surgical maneuvers (clipping, suturing, tying) it may be safer to leave behind a drain in attempting to prevent a hematoma (blood collection), if present, the drains will be removed within one to three days. Wear a soft comfortable bra for the first 7 days after surgery. Two weeks after surgery you may purchase any type of bra you wish. If non-absorbable sutures were used, they will be removed 7 days after surgery. You may begin breast massage the 2nd week after surgery, you will be instructed on breast massage at your post-op appointment, continue massaging for at least 6 weeks after surgery. Do not expose incisions to the sun and/or tanning UV light for at least 1 year, however, you may begin tanning 4 weeks after surgery while keeping incisions covered. If sun exposure in unavoidable, use a product with SPF of at least 30.
Who would be considered a good candidate for breast enlargement or breast augmentation surgery?
The ideal candidate is over 18 years of age, that is a consenting adult, psychologically fit, healthy, has well-defined and realistic expectations of the breast enlargement process, and is not under the impression that breast augmentation surgery is the answer to all of her life’s problems. Patients who are inappropriate candidates include women with a breast malignancy, or pre-cancerous tumor(s), are pregnant or nursing or breast feeding post-partum, the presence of acute or chronic infection, including oral or dental. Relative red flags include psychological or emotional instability, stressful life-events, and unrealistic goals and expectations.
What is capsular contracture after breast augmentation surgery, what are the consequences, and what is the treatment?
A breast implant capsule is the firm, sometimes hard and thick, scar tissue that forms on the inside of the breasts and surrounds the breast implants after breast enlargement surgery. This sphere can contract over time, and squeeze the breast implants (capsular contracture). This would certainly place the breast implants in one position with almost no mobility. The positions of the left and right implants may be distorted with respect to each other, and also in relation to the chest wall. The shape of the breasts may also be made abnormal in any direction. The breasts may also feel unnaturally hard. Finally, significant pain may also be present. The incidence of this capsular contracture is thought by many plastic surgeons to be related to blood collection, fluid collection, contamination, or infection at the time of breast enlargement surgery. It is also more common when the breast implants are placed on top rather than behind the pectoralis muscle. Surgical options for the treatment of capsular contracture include releasing the capsule circumferentially, completely excising the capsule, or even the former or latter combined with breast implant replacement. Regardless of the intervention, there is no guarantee against the recurrence of capsular contracture.
Is chest wall deformity common after breast enlargement surgery?
Chest wall deformity may be a result of aging, or the pressure exerted by a usually large breast implant in relation to the patient breast and chest wall size. This adverse effect is not very common.More common is a congenital anomaly whereby the deficit is present before augmentation as in pectus excavatum or carinatum (and inward or outward bowed chest).
Is chronic pain common after breast enlargement surgery?
Acute pain is common, chronic pain is not. Both U.S. implant manufacturers have similar data to this effect. Although “improper size, placement” are stated by some to be the culprits, the allegation is laughable, because the proper size and placement is dependent entirely upon patient anatomy and preferences. Transection of nerves supplying skin may lead to prolonged pain as the nerves heal over months. Most surgeons will attempt to stretch the tissue around the area where the nerve travels hoping to avoid this. Capsular contracture may cause traction on cutaneous nerves, lading to pain. Relentless, intense breast pain should prompt an urgent notification of the operating plastic surgeon.
What is closed capsulotomy?
The inside scar that forms around a breast implant, whether silicone or saline, can compress that implant and make it look and feel very unnatural. Years ago a method devised to “break” apart this capsule was quite commonly used. Although successful in some instances, many times the technique led to implant rupture, necessitating removal of the breast implants, and re-implantation.
Are gummy bear implants the final word in breast augmentation?
This question can be answered by comparing Rembrandt painting with his old brush, and a painter-for-hobby using the latest and greatest in paintbrush technology. It is not so much the brush, as the brusher. I’ll take Rembrandt any day. That being said, the so called “gummy bear” silicone breast implants have many advantages, and a few disadvantages. They are likely the ideal implant for breast reconstruction patients, patients with thin skin or no native breast tissue who desire large breasts, and patients to whom incision size is not of the outmost importance.
Can any surgeon use the cohesive gummy bear implant?
Any board certified plastic surgeon who has agreed to abide by the patient selection criteria, to provide follow-up data, and to enroll in the ongoing studies may use this implant.
Is there a way to detect cohesive gel (“gummy bear”) breast implant rupture or leak?
Presently, and as with other types of silicone breast implants, the cohesive gel (“gummy bear”) silicone breast implants can only be examined for a possible leak by Magnetic Resonance Imaging (MRI). Because silicone tends to remain inside the capsule that is formed around each implant, it would be very difficult to pick this up on routine x-rays or mammograms.
What happens if “Gummy Bear Breast Implants” tear, rupture, or leak?
After the implantation of thousands of such breast implants in Europe, the reported incidence of rupture is confined to several cases. This may be because most cases of rupture may not be picked up owing to the “gummy bear” consistency of the silicone gel. The consequences of leaking “gummy bear breast implants” are not well known, and there are studies going on right now to determine the safety of this cohesive silicone gel implant. Intuitively, the silicone “gummy bear” implant should not migrate as much as would be expected of the more liquid older generation silicone breast implants.
Why aren’t any Inamed or Mentor contoured or anatomically shaped silicone gummy bear breast implants made smooth?
Regardless of the degree of silicone cohesiveness in an anatomically shaped breast implant, it will always have a thinner end and a thicker end. It is very important that the position of the implant be maintained so that no distortion occurs, as would happen if the breast implant flipped up, or front to back. Textured implants tend to adhere firmly to their breast capsule, preventing any rotation, and malposition.
What are some of the complications of breast enlargement surgery?
Hemorrhage (bleeding), infection (superficial skin and soft tissue, or deep around the soft tissue surrounding the implant), implant exposure (implant poking through the incision), incision dehiscence (wound coming open), loss of nipple sensation (complete or partial), loss of sensation in breast skin (usually lower part), palpable or visible defects such as rippling or capsular contracture, breast implant rupture, silicone leak with the possibility of a local inflammatory reaction, capsular contracture (inside scar that may squeeze the implant into an unnatural form), seroma (fluid accumulation as your body attempts to fill a potential space).
Is there an association between breast augmentation surgery and connective tissue disease?
Pseudoscientific “studies” reported on cases of connective tissue disease supposedly associated with breast implant use in breast augmentation surgery. No study to date has ever demonstrated this. Many women who went on to develop connective tissue disease after breast augmentation attributed the problem to the implants, but the truth is that the incidence of such patients within the breast enlargement population and the general population is the same.
What other points should I consider when chosing silicone gel-filled breast implants to be used in my breast enlargement?
In addition to all of the possible complications, and unplanned additional surgeries you should also consider the possibility of health insurance premium increases. It may also be a good idea to invest in CosmetAssure®. This is an independent insurance company that offers coverage for patient treatment in cases of post-surgical complications related to cosmetic surgery for a one-time premium.
What is the customary cost of breast augmentation or breast enlargement surgery?
Prices range from as little as $2,500 to as high as $15,000. There are several factors that go into the price of breast enlargement surgery.Geographical area is probably the most likely factor to affect the price of breast enlargement surgery. Prices tend to run lower in regions where the population is less affluent on average, where cosmetic surgery is considered less necessary, but also where competition is more prevalent.Surgeon availability is another important factor in price setting. A surgeon whose schedule is entirely booked for months on end, will generally “skim the top,” in terms of patients who can afford higher prices. This does not mean there is no one out there who can do as good a job. It means that a particular surgeon knows he or she can get a certain amount of money for his or her breast augmentations.Another important part of the ultimate cost that is passed on to the patient is whether or not the plastic surgeon owns the facility at which the breast enlargement surgery is carried out. Although part of the facility fee may then go to the owning surgeon, it may be set substantially lower than a facility fee that would be paid to a third party such as a hospital or operating center when carrying out the breast enlargement surgery.A factor related to the facility fee is whether or not the surgeon prefers to use general anesthesia, or to employ the services of an anesthesiologist, or whether the plastic surgeon favors surgeon administered sedation with regional blocks for carrying out breast enlargement surgery. Obviously, the addition of an anesthesiologist or even a certified nurse anesthetist will add cost to breast augmentation. It is important to realize, however, that paying for an anesthesiologist does add a certain measure of safety, regardless of what anyone may tell you to the contrary.
Is dissatisfaction with cosmetic results common after breast augmentation surgery?
Both Allergan and Mentor publish data with respect to adverse outcomes after breast enlargement surgery with either silicone or saline breast implants. Disappointment with cosmetic outcomes of breast enlargement surgery with either silicone or saline breast implants are a result of miscommunication in preference or obvious defects. The former comes from the plastic surgeon and his or her patient not being on the same page with respect to shape, size, or incision placement. The latter is either an unavoidable fact of breast augmentation or a technical problem. Breast implant rippling and wrinkling, possible asymmetry with implant migration, scar migration, or audible sounds with manipulation that present months after the procedure are usually not the result of surgical misadventure. Asymmetry in shape, size, or incision placement immediately after the surgery can be attributed miscalculation. Hypertrophic scarring, implant visibility, implant palpability, and hardening of the breast capsule with resultant distortion in shape are typically not a result of surgical error.
What is “dual-plane” breast implant placement in breast augmentation?
The “dual-plane” approach allows placement of the upper portion of the implant under the muscle, and by releasing the lower portion of the muscle allows the lower portion of the breast implant to sit under the breast tissue. This eliminates the drawbacks of both the sub-glandular and sub-muscular placement while retaining the advantages of both. It is the most commonly performed placement in today’s breast augmentation surgery.
Is there a way to tell my own breast tissue from saline breast implants during self examination?
Distinguishing native breast tissue from saline breast implants is a necessary part of breast self-examination. It should be fairly obvious to discern breast from implant. If you encounter difficulties, ask your plastic surgeon to demonstrate the appropriate technique for breast self-examination and point out the difference between breast implants and breast tissue.
What are the restrictions to activity after breast enlargement surgery?
Dr. Gerzenshtein’s recommendations to his patients are found below. Consult with your own surgeon for his ore her specific instructions. Do not drive a car or engage in activities that depend on your coordination for 48 hours after your surgery, or after taking any of the pain, nausea or insomnia medications predscribed. Walking and getting about is highly encouraged for multiple reasons, including a decrease in the incidence of clot formation in the veins of your legs, have someone with you for the first 24 hours to monitor and help you get about as necessary. When resting/sleeping, lie on your back with several pillows under your head and back, or place a pillow or rolled blanket under the head of your mattress, this will decrease swelling. You may sleep on your side 2 weeks after surgery, and in any manner 4 weeks after surgery. Do not use your arms in strenuous activity such as vacuuming, pushing yourself up in bed, and pushing yourself up from sitting position for approximately 1 week. For the first week, avoid activities that raise your blood pressure such as heavy manual labor, repeated heavy lifting, strenuous exercise, or bending over. Plan to be away from work for one week, assuming your post-operative course is uncomplicated. After one week you may engage in light exercise only, such as walking. No heavy lifting should be done (10 lbs or more) for 4 weeks after breast augmentation. If weight training, refrain from upper body work for six weeks (substantial capsule formation), otherwise contraction of the pectoralis will push the implant up, changing the position of the final pocket to a more superior and less favorable position. Do not smoke, use nicotine substitutes (patches, chewing tobacco, etc.), and stay away from second hand smoke for at least 6 weeks after surgery. Smoking will decrease blood and oxygen flow to healing tissues and can cause loss (death) of skin, fat, and muscle in the operated field, especially along the incisions, slow down healing to double of normal time, worsen scar appearance on the outside, lead to a tough, fibrous scar on the inside, and increase the risk of fluid pockets.
What are the advantages and disadvantages to the use of cohesive “gummy bear” breast implants?
“Gummy Bear Breast Implants” have the consistency of gummy bears. This means there should be no folding or rippling. Being more formed should presumably allow the implants to maintain a shape closer to the original post-operative contour. Finally, should the implant rupture, migration should not occur to the same degree as with older generation implants.The main disadvantage to the cohesive “gummy bear” implants is that they require a somewhat larger incision to place. Being more formed does not allow for the kind of manipulation and squeezing through a tight incision that is possible with the older, more liquid-like silicone breast implants. The other drawback is that the gummy bear breast implants only come in the “teardrop,” contoured, or shaped variety in the U.S.
How and why do breasts shape change with age?
Three major factors accounting for significant shape and size changes within the breast need to be considered within each patient. A patient may have one or all three of the components acting to produce undesirable changes in the breasts. The factors are: Weight fluctuations, such as large weight-gain followed by a significant loss, stretching out the skin envelope, and then removing the contents (fat).Multiple pregnancies acting to increase the volume of breast tissue that pushes on the overlying skin envelope, and atrophies between periods of pregnancy.Loss of elasticity in the connective tissue scaffolding that keeps the skin from stretching too far. This may be related to aging, genetics, or both.
What are the various saline implants available from Allergan?

What are the various silicone implants available from Allergan?

I not happy with the size of my breasts, but I do not want breast enlargement surgery. What are my options?
There are three non-surgical options available to women who do not wish to undergo breast augmentation surgery. The first is to do nothing and concede to smaller size breasts. The second is to use a padded bra to give the appearance of larger breasts when clothed. The third, though not highly recommended, is to try using a vacuum type device over a several month course.
Should my physician use a shaped or round implant in performing my breast augmentation?
This decision depends as much on what the surgeon can accomplish using either the round or the anatomically contoured implants as on which type of implants to use.Assuming the breast pockets are dissected in to the inferior or lowermost extent in the exact same manner, the shaped implants will impart more fullness at the top, and provide a more natural, gently curving slope to the augmented breast. For this to happen, however, the lowermost portion of the dissection or pocket must be the same no matter which mammary implants you use. If a surgeon habitually dissects low enough inferiorly to drop round breast prosthesis low enough that upper pole fullness is lost, that surgeon will likely favor the teardrop shaped, or anatomically shaped implant to compensate for that dissection. For anatomically shaped or contoured implants to do what they were intended for, pocket dissection has to extremely precise. This is because contoured breast implants are not symmetric; they have a top and bottom. If the subglandular or submuscular pockets are too wide, the shaped implants can shift or even flip, imparting asymmetry and even worse an unnatural shape to the augmented breast. Even though shaped mammary implants are textured, this is still no guarantee against malposition. Finally using the round type of breast implants can impart more medial or inner breast fullness. This significantly improves cleavage, because more fill volume winds up toward the more central part of the breast.
What is an axillary incision in breast enlargement surgery?
Placing the incision in the axilla (underarm) allows for a smaller incision. It is useful when there is not much breast tissue present to begin with, and thus no prominent breast fold, and when the nipple-areola complex is small. It is not as well concealed as the periareolar incision, gives less control in terms of the ability to feel around the breast pockets in assuring symmetry, and placement of silicone breast implants will necessitate a larger incision which may be less pleasing.
Is bleeding common after breast augmentation surgery, what are the consequences, and what is the treatment?
Bleeding is not a common complication after breast augmentation surgery; however, it may lead to problems if not detected in a timely fashion. This applies more to acute hemorrhage immediately after breast enlargement surgery. The affected side would become considerably larger than the non-bleeding side, would possibly turn pale and purplish, and the resulting tension would potentially threaten the viability of the overlying skin. The volume of blood that a breast pocket could potentially accumulate is not large enough to be life-threatening unless combined with certain other conditions. The treatment for acute postoperative bleeding after breast augmentation surgery is an immediate return to the operating room, release of the collected blood, a search for the offending vessel, and control of the bleeder. A hematoma may form after breast augmentation surgery in the case of a slow bleed, not detected immediately, or with subsequent trauma, days, weeks, or months after the initial operation for breast enlargement. The result would be swelling, discoloration, and pain. If large, as judged by the operating surgeon, the collection would need drainage. Smaller hematomas can be absorbed by the body.
Where do breasts get their shape?
To understand how the breast is built, we must examine its scaffolding, and then add intervening components. Let’s pretend we are building breasts. First we’ll start by making the skin on the chest wall loose, in a manner of a conical tent. We will make it as loose as we need to fill it with the volume we desire. Then, we’ll build the scaffold. Picture branching lines of connective tissue (white, fibrous, scar like strands) that attach the covering of the chest wall muscle to the overlying skin. Now if you were to tent up on the loose skin in the center of the breast, the scaffold would only allow you to stretch to the limits of it length. This is what would limit the descent of your breasts via gravity, though eventually the scaffold would stretch, and cause sagging breasts. Tenting the scaffolding would also make spaces between its individual strands. The spaces will then be filled with breast parenchyma, or the breast tissue that is responsive to female hormones, produces secretions, swells with monthly cycles, and makes milk. Each segment of this breast tissue will then have a pipe to drain its secretions, or milk. The pipes would travel to the nipple, and get bigger, the nearer to the nipple they came as more pipes joined in from other parts of the breast, and would look like an upside down tree. Finally, a several centimeter layer of fat would be placed under the skin, between the breast parenchyma (tissue), and the skin. The thickness of the fatty padding would depend on the bodyfat composition of the recipient of our breast.
Will there be breast skin and breast tissue changes after silicone gel-filled breast implant use in breast augmentation surgery?
Breast implantation stretches the skin envelope of the breast. This can accentuate stretch marks. Over time, breast skin can thin, revealing breast implant irregularities and fold flaws. The additional weight can potentially lead to an increase in breast drooping, and necessitate breast lifting or tightening.
Is breast tissue atrophy common after breast enlargement surgery?
Breast tissue atrophy may be a result of aging, or the pressure exerted by a usually large breast implant in relation to the patient breast and chest wall size. This adverse effect is not very common.
Is it safe to breastfeed my child after silicone breast enlargement surgery?
After the scare tactics of certain aspiring stars in the media had been worn thin by legitimate studies, it became clear that breast feeding after silicone breast augmentation was safe. From a purely physiologic standpoint two factors stand in the way of silicone secretion into milk. The first factor is the size of the silicone at the molecular level, the second is the fact that silicone never comes into direct contact with the cells that line the duct that secretes the milk. One of the surgical approaches to breast augmentation surgery, the circumareolar (around nipple) cuts through the breast ducts. Three approaches, the inframammary (under breast in fold), transaxillary (armpit), and TUBA (through the belly button). Even when the surgical approach carries a risk of transecting, or cutting through the milk carrying ducts, the passages either heal, or become blocked as in fibrocystic change, but do not retain a communication with the implant cavity making this of no consequence. As much as it would make for a good sensationalistic “story,” silicone breast implants do not harm the baby.
Can calcium collections in the breast after saline or silicone breast implant surgery be mistaken for cancer?
Besides cancer, calcium accumulates in areas of trauma or inflammation such as surgical sites. Although calcium present in and/or around tumors has a distinct configuration, it may be mistaken for a possible malignancy with a resultant recommendation for biopsy and/or extirpative surgery. Such procedures may result in the need for implant removal and replacement, or even breast reconstruction. A baseline mammogram prior to breast augmentation , and radiologic centers experienced in the Eklund technique would likely diminish the chances for this.
Is there an association between breast augmentation surgery and cancer?
According to multiple studies, there is no association between breast enlargement surgery and breast cancer.
Am I a good candidate for saline-filled breast implants?
The ideal candidate is over 18 years of age, that is a consenting adult, psychologically fit, healthy, has well-defined and realistic expectations of the breast enlargement process, and is not under the impression that breast augmentation surgery is the answer to all of her life’s problems. Patients who are inappropriate candidates include women with a breast malignancy, or pre-cancerous tumor(s), are pregnant or nursing or breast feeding post-partum, the presence of acute or chronic infection, including oral or dental. Relative red flags include psychological or emotional instability, stressful life-events, and unrealistic goals and expectations.
What type of anesthesia is typically used to carry out breast augmentation or breast enlargement surgery?
The two options available to clients and surgeons in maintaining a comfortable, safe and painless environment for the tummy-tuck patient, and a controlled setting for the abdominoplasty surgeon performing the body contouring surgery are general anesthesia and intravenous sedation combined with local anesthesia. The advantages of general anesthesia include complete unawareness on the part of the patient during abdominoplasty, a secure airway, and a still, controlled environment for the operating surgeon. The greatest disadvantages are post-operative nausea and vomiting, risks associated with general anesthesia and post-operative lethargy that slows recovery. The advantages to sedation type anesthesia for tummy-tuck surgery are just the reverse of the disadvantages noted for general anesthesia; recovery is faster, nausea and vomiting are minimized, and the systemic risks associated with general anesthesia are abolished. It is not possible to perform abdominoplasty under sedation in all cases, however, the amount of the tissue excised typically dictating whether it may or may not be used.
Will I be able to breast feed after breast enlargement surgery?
Breast augmentation surgery should not affect breast feeding. Because breasts gain more projection and substance after enlargement, they are typically easier to hold. Because they protrude more, and are easier to hold, breast feeding becomes much easier. Paradoxically breast feeding is actually less difficult for some women, after breast augmentation surgery. Given all of this, however, there are studies showing that women with breast implants report an inability to feed in up to 2/3’s of the implanted population, compared to 7 in 100 for women without breast implants. It is doubtful, however, that matching for size and age such results would ever hold up.
Is delayed wound healing common after breast enlargement surgery?
Delayed wound healing is not very common after breast enlargement surgery. Because the incisions are relatively small, and with proper technique the breast tissue is not devascularized the problem is rarely seen in healthy patients. Nicotine use, poorly controlled diabetes, chemotherapy, radiation therapy, vascular disease, immunosuppressive therapy or disease, use of corticosteroids may all lead to delayed wound healing.
Is abscess formation common after breast augmentation surgery with silicone breast implants, what are the consequences, and what is the treatment?
Any surgery, in any discipline carries a risk of infection. The risk is calculated based on the degree of contamination for a particular operation. Breast augmentation is considered a “clean” surgery, and carries an overall infection rate of less than two percent. If infection should take place, it will most often affect one or more of three patterns, assuming there is no disseminated spread, and the infection remains localized. Infection can occur in the skin, in the soft tissue surrounding the implant, and in the form of a pus pocket. Skin infection will usually respond to oral antibiotics. Soft tissue infections surrounding the breast implant may respond to oral antibiotics, will sometimes require intravenous antibiotics, and in other cases need to be treated with implant removal. If the infection should progress to, or start out as an abscess (pus-pocket), the only treatment that will be effective in treating the infection and preventing more serious systemic complications is drainage of pus and breast implant removal. Toxic Shock Syndrome (TSS) may result from the presence of a foreign body (breast implant in this case) in the setting of an infection, and is a truly life-threatening condition that needs to be addressed immediately. It is marked by high fever, nausea and vomiting, diarrhea, light-headedness and possibly loss of consciousness, and a diffuse rash. The treatment is timely institution of IV antibiotics, and breast implant removal. If the breast implant is removed, the infection should be treated, the inflammation allowed to resolve, and a new implant placed weeks down the road.
When should silicone gel-filled breast implants not be used in breast enlargement surgery?
Absolute contraindications for the use of silicone breast implants are the same as for the use of saline. Breast implant surgery should not be performed in patients who have not received treatment for breast cancer, who are pregnant, nursing, or have active infection in any part of their body.
Sources
- American Society of Plastic Surgery: https://www.plasticsurgery.org/cosmetic-procedures/breast-augmentation
- American Society of Aesthetic Plastic Surgeons: https://www.smartbeautyguide.com/procedures/breast/breast-augmentation/